Summary
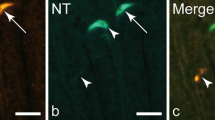
Ontogenetic appearances of glucagon, insulin and tyrosine hydroxylase (TH) were immunohistochemically investigated on developing pancreatic islets of rats. Glucagon immunoreactivity appeared first in some epithelial cells (g-cells) of the dorsal anlage of the pancreas on day 11.5 of gestation. On day 12.5, g-cells increased in number manufacturing the primitive islets, in which some cells appeared to be immunoreactive for insulin (i-cells) and about 40% of g-cells indicated also a slight immunoreactivity for insulin (g/i-cells). Afterwards, all the islet cells, especially g-cells, increased in number, and almost half of g-cells were g/i-cells. After day 16.5 of gestation, numerical increase of the cells with insulin immunoreactivity exceeded that of the cells with glucagon immunoreactivity, and about one fifth of g-cells were g/i-cells. After 20.5 days, however, no g/i cells were found. On day 16.5 of gestation, the immunoreactivity for TH appeared in occasional cells of the islets, but the cells did not show immunoreactivity for glucagon or insulin. It is concluded that the progenitor cells of the pancreatic islets appear to synthesize both glucagon and insulin by day 20.5 of gestation, but differentiate giving rise to mature A and B cells of adult isoets afterward.
Similar content being viewed by others
References
Beaupain D, Dieterlen-Lièvre F (1974) Etude immunocytologique de la différenciation du pancréas endocrine chez l'embryon de Poulet. II. Glucagon. Gen Comp Endocrinol 23:421–431
Bergroth V, Reitamo S, Konttinen YT, Lalla M (1980) Sensitivity and nonspecific staining of various immunoperoxidase techniques. Histochemistry 68:17–22
Daikoku S, Kawano H, Okamura Y, Tokuzen M, Nagatsu I (1986) Ontogenesis of immunoreactive tyrosine hydroxylase-containing neurons in rat hypothalamus. Dev Brain Res 28:85–98
Dieterlen-Lièvre F, Beaupain D (1974) Etude immunocytologique de la différenciation du pancréas endocrine chez l'embryon de Poulet. I. Îlots à insuline. Gen Comp Endocrinol 22:62–69
Dubois PM, Paulin C, Assan R, Dubois MP (1975) Evidence for immunoreactive somatostatin in the endocrine cells of human foetal pancreas. Nature 256:731–732
Fontaine J, Le Douarin NM (1977) Analysis of endoderm formation in the avian blastoderm by the use of quail-chick chimaeras. The problem of the neurectodermal origin of the cells of the APUD series. J Embryol Exp Morphol 41:209–222
Fujii S (1979) Development of pancreatic endocrine cells in the rat fetus. Arch Histol Jpn 42:467–479
Fukayama M, Ogawa M, Hayashi Y, Koike M (1986) Development of human pancreas. Immunohistochemical study of fetal pancreatic secretory proteins. Differentiation 31:127–133
Hökfelt T, Holets VR, Staines W, Meister B, Melander T, Schalling M, Schultzberg M, Freedman J, Björklund H, Olson L, Lindh B, Elfvin L-G, Lundberg JM, Lindgren JÅ, Samuelsson B, Pernow B, Terenius L, Post C, Everitt B, Goldstein M (1986) Coexistence of neuronal messengers — an overview. Prog Brain Res 68:33–70
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Le Douarin NM, Teillet MA (1973) The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol 30:31–48
Nakane PK, Pierce GB Jr (1967) Enzyme labeled antibodies for the light and electron microscopic localization of tissue antigens. J Cell Biol 33:307–318
Nishino T, Kodaira T, Shin S, Imagawa K, Yanaihara N, Shima K, Kumahara Y (1981a) Production of antisera to des Asn28 Thr29 Homoser27-glucagon; The development of radioimmunoassay for total glucagon-like immunoreactivity in human plasma. Endocrinol Jpn 28:419–427
Nishino T, Kodaira T, Shin S, Imagawa K, Shima K, Kumahara Y, Yanaihara C, Yanaihara N (1981b) Glucagon radioimmunoassay with use of antiserum to glucagon C-terminal fragment. Clin Chem 27:1690–1697
Pearse AGE (1969) The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem 17:303–313
Pearse AGE, Polak JM (1971a) Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut 12:783–788
Pearse AGE, Polak JM (1971b) Cytochemical evidence for the neural crest origin of mammalian ultimobranchial C cells. Histochemie 27:96–102
Pictet R, Rutter WJ (1972) Development of the embryonic endocrine pancreas. In: Steiner DF, Freinkel N (eds) Handbook of physiology, sect 7 vol 1. Am Physiol Soc, Washington DC, pp 25–66
Pictet RL, Clark WR, Williams RH, Rutter WJ (1972) An ultrastructural analysis of the developing embryonic pancreas. Dev Biol 29:436–467
Polak JM, Rost FWD, Pearse AGE (1971) Fluorogenic amine tracing of neural crest derivatives forming the adrenal medulla. Gen Comp Endocrinol 16:132–136
Schweisthal MR, Frost CC, Brinn JE (1975) Stains for A, B, and D cells in fetal rat islets. Stain Technol 50:161–170
Schweisthal MR, Frost CC, Brinn JE (1976) Ontogeny of four cell types in fetal rat islets using histochemical techniques. Acta Diabetol Lat 13:30–39
Shima K, Hirota M, Ohboshi C, Sato M, Nishino T (1987) Release of glucagon-like peptide 1 immunoreactivity from the perfused rat pancreas. Acta Endocrinol (Copenh) 114:531–536
Sternberger LA, Hardy PH Jr, Cuculis JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunohistochemistry. Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–333
Teitelman G, Lee JK (1987) Cell lineage analysis of pancreatic islet cell development: Glucagon and insulin cells arise from catecholaminergic precursors present in the pancreatic duct. Dev Biol 121:454–466
Teitelman G, Joh TH, Reis DJ (1981a) Transformation of catecholaminergic precursors into glucagon (A) cells in mouse embryonic pancreas. Proc Natl Acad Sci USA 78:5225–5229
Teitelman G, Joh TH, Reis DJ (1981b) Linkage of the brain-skingut axis: Islet cells originate from dopaminergic precursors. Peptides 2 [Suppl]:157–168
Yoshinari M, Daikoku S (1982) Ontogenetic appearance of immunoreactive endocrine cells in rat pancreatic islets. Anat Embryol (Berl) 165:63–70
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hashimoto, T., Kawano, H., Daikoku, S. et al. Transient coappearance of glucagon and insulin in the progenitor cells of the rat pancreatic islets. Anat Embryol 178, 489–497 (1988). https://doi.org/10.1007/BF00305036
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305036