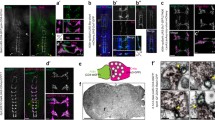
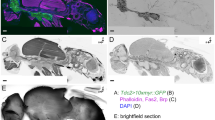
Abstract
Histamine is known to be the neurotransmitter of insect photoreceptors. Histamine-like immunoreactivity is also found in a number of interneurons in the central nervous system of various insects. Here, we demonstrate by immunohistochemical techniques that, in Drosophila melanogaster (Acalypterae), most or all mechanosensory neurons of imaginal hair sensilla selectively bind antibodies directed against histamine. The histamine-like staining includes the cell bodies of these neurons as well as their axons, which form prominent fibre bundles in peripheral nerves, and their terminal projections in the central neuropil of head and thoracic ganglia. The specificity of the immunostaining is demonstrated by investigating a Drosophila mutant unable to synthesize histamine. Other mechanosensory organs, such as campaniform sensilla or scolopidial organs, do not stain. In the calypteran flies, Musca and Calliphora, we find no comparable immunoreactivity associated with either hair sensilla or the nerves entering the central nervous system, observations in agreement with earlier studies on Calliphora. Thus, histamine seems to be a major mechanosensory transmitter candidate of the adult nervous system of Drosophila, but apparently not of Musca or Calliphora.
Similar content being viewed by others
References
Ashburner M (1989) Drosophila. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Blochlinger K, Jan LY, Jan YN (1991) Transformation of sensory organ identity by ectopic expression of cut in Drosophila. Genes Dev 5:1124–1135
Bodmer R, Bargel S, Sheperd S, Jack JW, Jan LY, Jan YN (1987) Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell 51:293–307
Buchner E (1991) Genes expressed in the adult brain of Drosophila and effects of their mutations on behaviour: a survey of transmitter-and second messenger-related genes. J Neurogenet 7:153–192
Buchner E, Buchner S, Crawford G, Mason WT, Salvaterra PM, Sattelle DB (1986) Choline acetyltransferase-like immunoreactivity in the brain of Drosophila melanogaster. Cell Tissue Res 246:57–62
Burg MG, Sarthy V, Pak WL (1991) Identification and cloning of a Drosophila gene homologous to rat histidine decarboxylase. Invest Ophthalmol Vis Sci 32:1151
Dambly-Chaudière C, Jamet E, Burri M, Bopp D, Basler K, Hafen E, Dumont N, Spielmann P, Ghysen A, Noll M (1992) The paired box gene pox neuro: a determinant of poly-innervated sense organs in Drosophila. Cell 69:159–172
Elias MS, Evans PD (1983) Histamine in the insect nervous system: distribution, synthesis and metabolism. J Neurochem 41:562–568
Hardie RC (1987) Is histamine a neurotransmitter in insect photoreceptors?. J Comp Physiol [A] 161:201–213
Hardie RC (1988) Effects of antagonists on putative histamine receptors in the first visual neuropile of the housefly (Musca domestica). J Exp Biol 138:221–241
Hardie RC (1989a) A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature 339:704–706
Hardie RC (1989b) Neurotransmitters of the compound eye. In: Stavenga DG, Hardie RC (eds) Facets of Vision, Springer, Berlin Heidelberg New York, pp 235–256
Hofbauer A, Buchner E (1989) Does Drosophila have seven eyes? Naturwissenschaften 76:335–336
Homberg U, Hildebrand JG (1991) Histamine-immunoreactive neurons in the midbrain and suboesophageal ganglion of the sphinx moth Manduca sexta. J Comp Neurol 307:647–657
McCaman RE, Weinreich D (1985) Histaminergic synaptic transmission in the cerebral ganglion of Aplysia. Neurophysiology 53:1016–1037
McClintock TS, Ache BW (1989) Histamine directly gates a chloride channel in lobster olfactory receptor neurons. Proc Natl Acad Sci USA 86:8137–8141
McIver SB (1985) Mechanoreception. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol 6. Nervous system: sensory. Pergamon Press, Oxford, pp 71–132
Merritt DJ, Murphey RK (1992) Projections of leg proprioceptors within the CNS of the fly Phormia in relation to the generalized insect ganglion. J Comp Neurol 322:16–34
Murphey RK, Possidente DR, Vandervorst P, Ghysen A (1989a) Compartments and the topography of leg afferent projections in Drosophila. J Neurosci 9:3209–3217
Murphey RK, Possidente D, Pollack G, Merritt DJ (1989b) Modality-specific axonal projections in the CNS of the flies Phormia and Drosophila. J Comp Neurol 290:185–200
Nässel DR (1991) Neurotransmitters and neuromodulators in the insect visual system. Prog Neurobiol 37:179–254
Nässel DR, Elekes K (1992) Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res 267:147–167
Nässel DR, Holmqvist MH, Hardie RC, Håkanson R, Sundler F (1988) Histamine-like immunoreactivity in photoreceptors of the compound eyes and ocelli of the flies Calliphora erythrocephala and Musca domestica. Cell Tissue Res 253:639–646
Nässel DR, Pirvola U, Panula P (1990) Histamine-like immunoreactive neurons innervating putative neurohaemal areas and central neuropil in the thoraco-abdominal ganglia of the flies Drosophila and Calliphora. J Comp Neurol 297:525–536
Nottebohm E, Dambly-Chaudière C, Ghysen A (1992) The connectivity of chemosensory neurons is controlled by the gene poxn in Drosophila. Nature 359:829–832
Pirvola U, Tuomisto L, Yamatodani A, Panula P (1988) Distribution of histamine in the cockroach brain and visual system: an immunocytochemical and biochemical study. J Comp Neurol 276:514–526
Pollack I, Hofbauer A (1991) Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res 266:391–398
Power ME (1948) The thoracico-abdominal nervous system of an adult insect, Drosophila melanogaster. J Comp Neurol 88:347–409
Restifo LL, White K (1990) Molecular and genetic approaches to neurotransmitter and neuromodulator systems in Drosophila. Adv Insect Physiol 22:115–219
Sarthy V, Burg MG, Pak WL (1991) A transient-deficient ERG mutant is blocked in histamine synthesis in Drosophila photoreceptors. Invest Ophthalmol Vis Sci 32:1266
Schlemermeyer E, Schütte M, Ammermüller J (1989) Immunohistochemical and electrophysiological evidence that locust ocellar photoreceptors contain and release histamine. Neurosci Lett 99:73–78
Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M (1991) Histaminergic transmission in the mammalian brain. Physiol Rev 71:1–51
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Buchner, E., Buchner, S., Burg, M.G. et al. Histamine is a major mechanosensory neurotransmitter candidate in Drosophila melanogaster . Cell Tissue Res 273, 119–125 (1993). https://doi.org/10.1007/BF00304618
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00304618