Summary
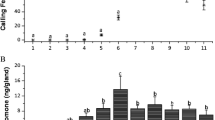
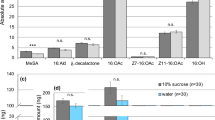
Males of Utetheisa ornatrix have a pair of brushlike glandular structures, the coremata, which they evert from the abdomen during close-range precopulatory interaction with the female. Males experimentally deprived of coremata are less acceptable to females. The principal chemical associated with the coremata, identified as a pyrrolizine (hydroxydanaidal), has a proven pheromonal role: males raised under conditions where they fail to produce hydroxydanaidal are also less likely to succeed in courtship, and the compound itself, as its (-)-isomer, is capable of inducing the principal receptive response (wing raising) of the female. Evidence is presented indicating that Utetheisa derive hydroxydanidal from defensive pyrrolizidine alkaloids that they sequester from their larval foodplants (Crotalaria spp.). It is proposed that in addition to signalling male presence to the female, hydroxydanaidal may provide the means whereby the female assesses the alkaloid content of the male and therefore his degree of chemical protectedness. The argument is made that such pheromonal assessment of defensive capacity may occur also in other insects, including danaid butterflies, many of which share with Utetheisa a dependence on pyrrolizidine alkaloids for sex-pheromone production.
Similar content being viewed by others
References
Adams R, Rogers EF (1939) The structure of monocrotaline, the alkaloid in Crotalaria spectabilis and Crotalaria retusa. J Am Chem Soc 61:2815–2819
Attenburrow J, Cameron AFB, Chapman JH, Evans RM, Hems BA, Jansen ABA, Walker J (1952) A synthesis of vitamin A from cyclohexanone. J Chem Soc 1094–1111
Barth R (1960) Orgaos odoriferos dos Lepidopteros. Bol Parque nac Italia 7:1–159
Bettini S (1978) Arthropod venoms. In: Handbuch der Experimentellen Pharmakologie, vol 48. Springer, Berlin Heidelberg New York
Boppré M (1979) Chemical communication, plant relationships, and mimicry in the evolution of danaid butterflies. Entomol Exp Applic 24:264–277
Bull LB, Culvenor CCJ, Dick AT (1968) The pyrrolizidine alkaloids. North Holland, New York
Conner WE, Masters WM (1978) Infrared video viewing. Science 199:1004
Conner WE, Eisner T, Vander Meer, RK, Guerrero A, Ghiringelli D, Meinwald J (1980) Sex attractant of an arctiid moth (Utetheisa ornatrix): a pulsed chemical signal. Behav Ecol Sociobiol 7:55–63
Conover WJ (1971) Practical nonparametric statistics. Wiley, New York
Culvenor CCJ (1970) Toxic plants—a re-evaluation. Search 1:103–110
Culvenor CCJ, Edgar JA (1972) Dihydro-pyrrolizine secretions associated with coremata of Utetheisa moths (Family Arctiidae). Experientia 28:627–628
Edgar JA, Culvenor CCJ, Robinson GS (1973) Hairpencil dihydropyrrolizine derivatives of Danaidae from the New Hebrides. J Aust Entomol Soc 12:144–150
Ehrlich PR, Raven PH (1965) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Eisner T (1970) Chemical defense against predation in arthropods. In: Sondheimer E, Simeone JB (eds) Chemical ecology. Academic Press, New York, pp 157–217
Eisner T (1980) Chemistry, defense, and survival: case studies and selected topics. In: Locke M, Smith DS (eds) Insect biology in the future. Academic press, New York, pp 847–878
Menschikoff G (1932) Über die Alkaloide von Heliotropium lasiocarpum. Ber Dtsch Chem Ges 65:974–977
Miller JR, Baker TC, Cardé RT, Roelofs WL (1976) Reinvestigation of oak leaf roller sex pheromone components and the hypothesis that they vary with diet. Science 192:140–142
Pease RW Jr (1968) Evolution and hybridization in the Utetheisa ornatrix complex (Lepidoptera: Arctiidae). I. Inter- and intrapopulation variation and its relation to hybridization. Evolution 22:719–735
Pliske TE, Eisner T (1969) Sex pheromone of the queen butterfly: biology. Science 164:1170–1172
Sawhney RS, Girotia RN, Atal CK, Culvenor CCJ, Smith LW (1967) Phytochemical studies on genus Crotalaria: Part VII-Major alkaloids of C. mucronata, C. brevifolia and C. laburnifolia. Indian J Chem 5:655–656
Schneider D, Boppré M, Schneider H, Thompson WR, Boriack, CJ, Petry RL, Meinwald J (1975) A pheromone precursor and its uptake in male Danaus butterflies. J Comp Physiol 97:245–256
Sustare DB (1978) Systems diagrams. In: Colgan PW (ed) Quantitative ethology. Wiley, New York, pp 275–311
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Conner, W.E., Eisner, T., Vander Meer, R.K. et al. Precopulatory sexual interaction in an arctiid moth (Utetheisa ornatrix): Role of a pheromone derived from dietary alkaloids. Behav Ecol Sociobiol 9, 227–235 (1981). https://doi.org/10.1007/BF00302942
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00302942