Abstract
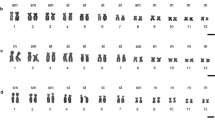
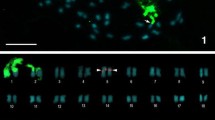
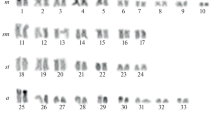
The order Monotremata, comprising the platypus and two species of echidna (Australian and Nuigini) is the only extant representative of the mammalian subclass Prototheria, which diverged from subclass Theria (marsupials and placental mammals) 150–200 million years ago. The 2n=63♂, 64♀ karyotype (newly described here) of the Nuigini echidna is almost identical in morphology and G-band pattern to that of the Australian echidna, from which it diverged about a million years ago. The karyotype of the platypus (2n=52) has several features in common with those of the echidna species; six pairs of large autosomes, many pairs of small (but not micro-) chromosomes, and a series of small unpaired chromosomes which form a multivalent at meiosis. Comparison of the G-band patterns of platypus and echidna autosomes reveals considerable homology. Chromomycin banding demonstrates GC-rich heterochromatin at the centromeres of many platypus and echidna chromosomes, and at the nucleolar organizing regions; some of this heterochromatin C-bands weakly in platypus (but not echidna) spreads. Late replication banding patterns resemble G-banding patterns and confirm the homologies between the species. Striking heteromorphism between chromosomes of some of the large autosomal pairs can be accounted for in the echidna by differences in amount of chromomycin-bright, late replicating heterochromatin. The sex chromosomes in all three species also bear striking homology, despite the difference in sex determination mechanism between platypus (XX/XY) and the echidna species (X1X1X2X2/X1X2Y). The platypus X and echidna X1 each represent about 5.8% of haploid chromosome length, and are G-band identical. Y chromosomes are similar between species, and are largely homologous to the X (or X1).
Similar content being viewed by others
References
Archer M, Flannery TF, Ritchie A, Molnar RE (1985) First Mesozoic mammal from Australia — an early Cretaceous monotreme. Nature 318:363–366
Bick YAE, Jackson WD (1967a) A mammalian X-O sex-chromosome system in the monotreme Tachyglossus aculeatus determined from leucocyte cultures and testicular preparations. Am Nat 101:79–86
Bick YAE, Jackson WD (1967b) Karyotype of the monotremes Ornithorhynchus anatinus (platypus) and Tachyglossus aculeatus (echidna), Nature 214:600–601
Bick YAE, Sharman GB (1975) The chromosomes of the platypus (Ornithorhynchus:Monotremata). Cytobios 14:17–28
Bick YAE, Murtagh CE, Sharman GB (1973) The chromosomes of an egg-laying mammal, Tachyglossus aculeatus (the echidna). Cytobios 7:233–243
Bloom SE, Goodpasture C (1976) An improved technique for selective silver staining of nucleolar organizer regions in human chromosomes. Hum Genet 34:199–206
Dawson GW, Graves JAM (1984) Gene mapping in marsupials and monotremes. I. The chromosomes of rodent-marsupial (Macropus) cell hybrids, and gene assignments to the X chromosome of the grey kangaroo. Chromosoma 91:20–27
Dutrillaux B (1979) Chromosomal evolution in primates: tentative phylogeny from Microcebus murinus (prosimian) to man. Hum Genet 48:251–314
Eichenbaum S, Krumins E (1983) A simple and reliable method of chromosome banding for prenatal cytogenetics using a bromodeoxyuridine pulse. Prenat Diagn 3:291–296
Graves JAM (1987) Marsupial and monotreme gene maps. In: O'Brien SJ (ed) Genetic maps 4. Cold Spring Harbor Laboratory Press, NY, pp 501–504
Hopson JA (1970) The classification of non-therian mammals. J Mamm 51:1–9
Human Gene Mapping 8 (1985) Eighth International Workshop of Human Gene Mapping. Cytogenet Cell Genet 40
Kemp TS (1983) The relationships of mammals. Zool J Linn Soc 77:353–384
Kodama Y, Yoshida MC, Sasaki M (1980) An improved silver staining technique for nucleolus organizer regions by using nylon cloth. Jpn J Hum Genet 25:229–233
Leversha M, Sinfield C, Webb G (1980) Rapid and reliable methods for the G- and C-banding of human and other mammalian chromosomes. Aust J Med Lab Sci 1: 139–143
Matthey R (1949) Les chromosomes des vertebrees. F. Rouge, Lucerne
Murray P (1984) Furry egg-layers — The monotreme radiation. In: Archer M, Clayton G (eds) Vertebrate zoogeography and evolution in Australasia. Hesperian Press, Western Australia
Murtagh CE (1977) A unique cytogenetic system in monotremes. Chromosoma 65:37–57
Murtagh CE (1978) Cytogenetics of the monotremes — sex chromosomes, chromosome polymorphisms and dose compensation for X chromosomes. M.Sc. thesis, Macquarie University, North Ryde, Australia
Nash WG, O'Brien SJ (1982) Conserved regions of homologous G-banded chromosomes between orders in mammalian evolution: carnivores and primates. Proc Natl Acad Sci USA 79:6631–6635
Ohno S (1967) Sex chromosomes and sex linked genes. Springer, Berlin
Olert J (1979) Interphase studies with a simplified method of silver staining of nucleoli. Experientia 35:283–285
Rofe R, Hayman D (1985) G-banding evidence for a conserved complement in the marsupialia. Cytogenet Cell Genet 39:40–50
Schweizer D (1976) Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58:307–324
Schweizer D (1981) Counter stain-enhanced chromosome banding. Hum Genet 57:1–14
Van Brink J (1959) L'expression morphologique de la diagametie chez les sauropsides et les monotremes. Chromosoma 10:1–72
White MJD (1973) Animal cytology and evolution, 3rd edn. Cambridge University Press
Whittacker RG, Thompson EOP (1974) Studies on monotreme proteins. V. Amino acid sequences of the α-chain of haemoglobin from the platypus, Ornithorhynchus anatinus. Aust J Biol Sci 27:591–605
Wrigley JM, Graves JAM (1984) Two monotreme cell lines, derived from female platypuses (Ornithorhynchus anatinus; Monotremata, Mammalia). In Vitro 20:321–328
Wrigley JM, Graves JAM (1988 a) Sex chromosome homology and incomplete, tissue-specific X inactivation suggests that monotremes represent an intermediate stage of mammalian sex chromosome evolution. J Hered, in press
Wrigley JM, Graves JAM (1988b) Gene mapping in marsupials and monotremes. V. Synteny between HPT and PGK in the platypus. Aust J Biol Sci, in press
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wrigley, J.M., Graves, J.A.M. Karyotypic conservation in the mammalian order monotremata (subclass Prototheria). Chromosoma 96, 231–247 (1988). https://doi.org/10.1007/BF00302363
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00302363