Summary
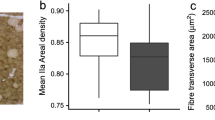
Fiber composition, and glycolytic and oxidative capacities of the pectoralis, gastrocnemius, and cardiac muscles from active and hibernating little brown bats (Myotis lucifugus) was studied. The data were used to test two hypotheses: First, since hibernating bats maintain the capability of flight and make use of leg muscles to maintain a roosting position all winter, the fiber composition of the pectoralis and gastrocnemius muscles should not change with season. Second, we tested the hypothesis of Ianuzzo et al. (in press), who propose that the oxidative potential of mammalian cardiac muscle should increase with increasing heart rate while glycolytic potential should not. Our results indicate that the fiber composition of the pectoralis muscle was uniformly fast-twitch oxidative (FO)_ regardless of the time of year, as predicted. However, the gastrocnemius muscle exhibited a change in FO composition from 83% in active to 61% in hibernating animals. Contrary to the variable change in histochemical properties with metabolic state, a trend of reduced maximal oxidative (CS) and glycolytic (PFK) potential during hibernation in both flight and leg muscles was apparent. The oxidative potential of flight and leg muscles decreased by 15.2% and 56.5%, respectively, while the glycolytic potential of the same muscles decreased by 23.5% and 60.5%, respectively. As predicted, the glycolytic potential of cardiac muscle remained constant between active and hibernating bats, although there was a significant decrease (22.0%) in oxidative potential during hibernation.
Similar content being viewed by others
Abbreviations
- FO :
-
fast-twitch oxidative
- FG :
-
fast-twitch glycolytic
- SO :
-
slow-twitch oxidative
- Vmax :
-
maximal enzyme activity
- PFK :
-
phosphofructokinase
- CS :
-
citrate synthase
References
Armstrong RB, Ianuzzo CD, Kunz TH (1977) Histochemical and biochemical properties of flight muscle fibers in the little brown bat, Myotis lucifugus. J Comp Physiol 119:141–154
Barclay RMR (1982) Night roosting behaviour of the little brown bat, Myotis lucifugus. J Mamm 63:464–474
Barclay RMR (1985) Foraging behaviour of the African insectivorous bat, Scotophilus leucogaster. Biotropica 17:65–70
Blank S, Chen V, Hamilton N, Salerno TA, Ianuzzo CD (1989) Biochemical characteristics of mammalian Myocardia. J Mol Cell Cardiol 21:367–373
Borgmann AI, Moon TW (1976) Enzymes of the normothermic and hibernating bat, Myotis lucifugus: Temperature as a modulator of pyruvate kinase J Comp Physiol 107:185–199
Brabec MJ, McColloch RJ (1970) Phosphofructokinase activity in the brown fat, liver, and heart of hibernating and homeothermic thirteen-lined ground squirrels (Citellus tridecemlineatus). Int J Biochem 1:557–560
Brigham RM, Fenton MB (1986) The influence of roost closure on the roosting and foraging behaviour of Eptesicus fuscus (Chiroptera: Vespertilionidae). Can J Zool 64:1128–1133
Davis WH, Reite OB (1967) Responses of bats from temperate regions to changes in ambient temperature. Biol Bull 132:320–328
Fenton MB (1969) Summer activity of Myotis lucifugus (Chiroptera: Vespertilionidae) at hibernacula in Ontario and Quebec. Can J Zool 47:597–602
Fenton MB (1970) Population studies of Myotis lucifugus (Chiroptera: Vespertilionidae) in Ontario. ROM Life Sci Contr 77 34pp
Fenton MB, Brigham RM, Mills AM, Rautenbach IL (1985) The roosting and foraging areas of Epomophorous wahlbergi (Pteropodidae) and Scotophilus viridis (Vespertilionidae) in Krüger National Park, South Africa. J Mamm 66:461–468
Fenton MB, Rautenbach IL (1986) A comparison of the roosting and foraging behaviour of three species of African insectivorous bats (Rhinolophidae, Vespertilionidae, Molossidae). Can J Zool 64:2860–2867
Foehring RC, Hermanson JW (1984) Morphology and histochemistry of flight muscles in free-tailed bats, Tadarida brasiliensis. J Mamm 65:388–394
Guth L, Samaha FJ (1970) Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol 28:365–367
Henshaw RE 1970 Thermoregulation in bats. In: Slaughter BH, Walton DW (eds) About bats. Southern Methodist University Press, Dallas, pp 188–232
Hurst RN (1966) Seasonal physiological responses of the bat Myotis lucifugus. Unpublished PhD Thesis, Purdue University, Lafayette Indiana
Ianuzzo CD, Gollnick PD, Armstrong RB (1976) Histochemical adaptations of rat skeletal muscle fibers to a long-term power overload condition. Life Sci 19:1517–1524
Ianuzzo CD, Blank S, Hamilton N, O'Brien P, Chen V, Brotherton S, Salerno TA (in press) The relationship of myocardial chronotropism to the biochemical capacities of mammalian hearts. Biochemistry of Exercise VII. Human Kinetics Publishers, Champaign Illinois
Moon TW (1978) Enzymes of heterotherms: LDH of hibernating and normothermic little brown bats, Myotis lucifugus. Comp Biochem Physiol 59B:183–190
Nicoll PA (1964) Arterial pressures in the bat Myotis lucifugus. Fed Proc Fed Am Soc Exp Biol 23:253
Novikoff AB, Shin W, Drucker J (1961) Mitochondrial localization of oxidative enzymes: staining results with two tetrazolium salts. J Biophys Biochem Cytol 9:47–61
Padykula HA, Herman E (1955) The specificity of the histochemical methods of adenosine triphosphatase. J Histochem Cytochem 3:170–195
Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE (1972) Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochem 11:2627–2634
Pette D (1984) Activity-induced fast to slow transitions in mammalian muscle. Med Sci in Sports Exer 16:517–528
Pette D, Vrbova G (1985) Neural control of phenotypic expression in mammalian muscle fibers. Muscle and Nerve 8:676–689
Racey PA (1974) Ageing and assessment of reproductive status of Pipistrelle bats, Pipistrellus pipistrellus. J Zool Lond 173:264–271
Saltin B, Gollnick PD (1983) Skeletal muscle adaptability: Significance for metabolism and performance. In: Peachy LD, Adrian RH, Geiger SR (eds) Handbook of physiology. Sec 10 Skeletal muscle Bethesda: Amer Physiol Sci pp 555–632
Shonk CE, Boxer GE (1964) Enzyme patterns in human tissue. I. method for determination of glycolytic enzymes. Cancer Res 24:709–721
Speakman JR, Racey PA (1989) Hibernal ecology of the Pipistrelle bat: Energy expenditure, water requirements and mass loss, implications for survival and the function of winter emergence flights. J Anim Ecol 58:797–813
Srere PA (1969) Citrate synthase. Meth Enzymol 13:3–8
Studier EH, O'Farrell JJ (1976) Biology of Myotis thysanodes and M. lucifugus (Chiroptera: Vespertilionidae). III. Metabolism, heart rate, breathing rate, evoporative water loss and general energetics. Comp Biochem Physiol 54:423–432
Thomas SP (1975) Metabolism during flight in two species of bats, Phyllostomus hastatus and Pteropus gouldii. J Exp Biol 63:273–293
Thomas DW, Fenton MB, Barclay RMR (1979) Social behaviour of the little brown bat, Myotis lucifugus. I. Mating behaviour. Behav Ecol Sociobiol 6:129–136
Wattenberg LW, Leong JL (1960) Effects of coenzyme Q10 and menadione on succinate dehydrogenase activity as measured by tetrazolium salt reduction. J Histochem Cytochem 8:296–303
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brigham, R.M., Ianuzzo, C.D., Hamilton, N. et al. Histochemical and biochemical plasticity of muscle fibers in the little brown bat (Myotis lucifugus). J Comp Physiol B 160, 183–186 (1990). https://doi.org/10.1007/BF00300951
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00300951