Abstract
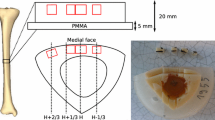
Measurements of the speed-of-sound (SOS) and of the broadband ultrasound attenuation (BUA) on the os calcis were recently proposed to assess osteoporotic fragility. Velocity and attenuation were measured through the heel which can be divided in three phases including hydroxyapatite, soft tissue, and fat. The aim of this study was to evaluate the influence of fat composition and heel width on SOS and BUA. This influence was determined from both in vitro investigations examining fat samples, phantoms, and cadaver heels, and in vivo ones observing adult volunteers as well as a wide sample section of healthy elderly women. Ultrasound velocities on various fat samples were significantly lower than those on distilled water (-65 m/second to -123 m/second). The excision of the surrounding soft tissue from cadaver heels made SOS steadily increase whereas the insertion of a 10 mm piece of lard in the lateral face of cadavers' and volunteers' heels os calcis lowered SOS about 30 m/second. ond. Furthermore, a difference of SOS was estimated at 15 m/second for a 12.5% variation of the marrow fat weight. Among 334 elderly and healthy women aged 75 and over, a significant negative correlation was found between SOS and heel width (r=-0.27; P<0.0001). On the other hand, fat composition had no significant effect on BUA measurement, and no significant relationship was found between BUA and heel width. This study demonstrates that an increase of heel width and fat thickness provides an underestimation of os calcis SOS, but has no significant effect on BUA.
Similar content being viewed by others
References
Mazess RB, Barden H, Ettinger M, Schultz E (1988) Bone density of the radius, spine, and proximal femur in osteoporosis. J Bone Miner Res 3:13–18
Mazess RB, Collick B, Trempe J, Barden H, Hanson J (1989) Performance evaluation of a dual-energy X-ray bone densitometer. Calcif Tissue Int 44:228–232
Cummings SR, Black DM, Nevitt MC (1990) Appendicular bone density and age prediction hip fracture in women. JAMA 263:665–668
Langton CM, Palmer SB, Porter RW (1984) The measurement of broadband ultrasonic attenuation in cancellous bone. Eng Med 13:89–91
Turner CH, Eich M (1991) Ultrasonic velocity as a predictor of strength in bovine cancellous bone. Calcif Tissue Int 49:116–119
Biot MA (1962) Generalized theory of acoustic propagation in porous dissipative media. J Acoust Soc Am 34:1254–1264
McCloskey EV, Murray SA, Charlesworth D, Miller C, Fordham J, Clifford K, Atkins R, Kanis JA (1990) Assessment of broadband ultrasound attenuation in the os calcis in vitro. Clin Sci 78:221–227
Bradenburger G, Waud K, Baran D (1992) Reproducibility of uncorrected velocity of sound does not indicate true precision. J Bone Miner Res (suppl 1):S184
Mazess B, Vetter J, Weaver DS (1987) Bone change in oophorectomized monkeys: CT findings. J Comput Assist Tomogr 11:302–305
White DR (1978) Tissue substitutes in experimental radiation physics. Med Phys 5:467–478
Zagzebski JA, Rossman PJ, Mesina C, Mazess RB, Madsen EL (1991) Ultrasound transmission measurements through the os calcis. Calcif Tissue Int 49:107–111
Greespan M, Tschigg CE (1959) Table of speed of sound in water. J Acoust Soc Am 31:75–76
Schott AM, Hans D, Sornay-Rendu E, Delmas PD, Meunier PJ (1993) Ultrasound measurements on os calcis: precision and age-related changes in a normal female populations. Osteoporosis Int 3:249–254
Goss SA, Johnston RL, Dunn F (1978) Comprehensive compilation of emprirical ultrasonic properties of mammalian tissues. J Acoust Soc Am 64:423–457
Johnston RL, Goss SA, Maynard V, Brady JK, Frizzell LA, O'Brien WD Jr, Dunn F (1979) Elements of tissue characterization, part I: ultrasonic propagation properties. In: Linzer M (ed) Ultrasonic tissue characterization II. NBS Special Publication 525, U.S. Government Printing Office, Washington, DC pp 19–27
Bamber JC (1983) Ultrasonic propagation properties of the breast. In: Jellins J, Kobayashi T (eds) Ultrasonic examination of the breast. John Wiley and Sons, Chichester pp 37–44
Jonson R, Manson LG, Rungren Å, Szück J (1990) Dual-photon absorptiometry for determination of bone mineral content in the calcaneus with correction for fat. Phys Med Biol 7:961–969
Rossman PJ, Zagzebski JA, Mesina C, Sorenson J, Mazess RB (1989) Comparison of speed of sound and ultrasonic attenuation in the os calcis to photon absorptiometry measurements in the radius, femur and lumbar spine. Clin Phys Physiol Meas 10:353–360
Agren M, Karellas A, Leahey D, Marks S, Baran D (1991) Ultrasound attenuation of the calcaneus: a sensitive and specific discriminator of osteopenia in postmenopausal women. Calcif Tissue Int 48:240–244
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kotzki, P.O., Buyck, D., Hans, D. et al. Influence of fat on ultrasound measurements of the Os calcis. Calcif Tissue Int 54, 91–95 (1994). https://doi.org/10.1007/BF00296057
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00296057