Abstract
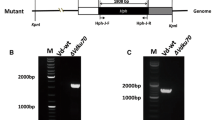
The feasibility of performing routine transformation-mediated mutagenesis in Glomerella cingulata was analysed by adopting three one-step gene disruption strategies targeted at the pectin lyase gene pnIA. The efficiencies of disruption following transformation with gene replacement- or gene truncation-disruption vectors were compared. To effect replacement-disruption, G. cingulata was transformed with a vector carrying DNA from the pnlA locus in which the majority of the coding sequence had been replaced by the gene for hygromycin B resistance. Two of the five transformants investigated contained an inactivated pnlA gene (pnlA −);both also contained ectopically integrated vector sequences. The efficacy of gene disruption by transformation with two gene truncation-disruption vectors was also assessed. Both vectors carried a 5′and 3′truncated copy of the pnlA coding sequence, adjacent to the gene for hygromycin B resistance. The promoter sequences controlling the selectable marker differed in the two vectors. In one vector the homologous G. cingulata gpdA promoter controlled hygromycin B phosphotransferase expression (homologous truncation vector), whereas in the second vector promoter elements were from the Aspergillus nidulans gpdA gene (heterologous truncation vector). Following transformation with the homologous truncation vector, nine transformants were analysed by Southern hybridisation; no transformants contained a disrupted pnlA gene. Of nineteen heterologous truncation vector transformants, three contained a disrupted pnlA gene; Southern analysis revealed single integrations of vector sequence at pnlA in two of these transformants. pnlA mRNA was not detected by Northern hybridisation in pnlA-transformants. pnlA-transformants failed to produce a PNLA protein with a pI identical to one normally detected in wild-type isolates by silver and activity staining of isoelectric focussing gels. Pathogenesis on Capsicum and apple was unaffected by disruption of the pnlA gene, indicating that the corresponding gene product, PNLA, is not essential for pathogenicity. Gene disruption is a feasible method for selectively mutating defined loci in G. cingulata for functional analysis of the corresponding gene products.
Similar content being viewed by others
References
Apel PC, Panaccione DG, Holden FR, Walton JD (1993) Cloning and targeted gene disruption of XYL1, a β1,4-xylanase gene from the maize pathogen Cochliobolus carbonum. Mol PlantMicrobe Interact 6:467–473
Brown AE, Adikaram NKB (1982) The differential inhibition of pectic enzymes from Glomerella cingulata and Botrytis cinerea by a cell wall protein from Capsicum annum fruit. Phytopath Z 105:27–38
Cervone F, Hahn MG, De Lorenzo G, Darvill A, Albersheim P (1989) Host-pathogen interactions. XXXIII. A plant protein converts a fungal pathogenesis factor into an elicitor of plant defense responses. Plant Physiol 90:542–548
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Cornelissen BJC, Melchers LS (1993) Strategies for control of fungal disease with transgenic plants. Plant Physiol 101:709–712
Desjardins AE, Hohn TM, McCormick SP (1992) Effect of gene disruption of trichodiene synthase on the virulence of Gibberella pulicaris. Mol Plant-Microbe Interact 5:214–222
Fincham JRS (1989) Transformation in fungi. Microbiol Rev 53:148–170
Fotheringham S, Holloman WK (1989) Cloning and disruption of Ustilago maydis genes. Mol Cell Biol 9:4052–4055
Hohn TM, Desjardins AE (1992) Isolation and gene disruption of the Tox5 gene encoding trichodiene synthase in Gibberella pulicaris. Mol Plant-Microbe Interact 5:249–256
Jeffries P, Dodd JC, Jeger MJ, Plumbley RA (1990) The biology and control of Colletotrichum species on tropical fruit crops. Plant Pathol 39:343–366
Keon JPR, Byrde RJW, Cooper RM (1987) Some aspects of fungal enzymes that degrade plant cell walls. In: Pegg GF, Ayres PG (eds) Fungal infection of plants. Symposium of the British Mycological Society. Cambridge University Press, Cambridge, UK, pp 133–157
Kronstad JW, Leong SA (1990) The b mating-type locus of Ustilago maydis contains variable and constant regions. Genes Dev 4:1384–1395
Kronstad JW, Wang J, Covert SF, Holden DW, McKnight GL, Leong SA (1989) Isolation of metabolic genes and demonstration of gene disruption in the phytopathogenic fungus Ustilago maydis. Gene 79:97–106
Liao C-H, Hung HY, Chategee AK (1988) An extracellular pectate lyase is the pathogenicity factor of the soft-rotting bacterium Pseudomonas viridiflava. Mol Plant-Microbe Interact 1:199–206
Marmeisse R, van den Ackerveken GFJM, Goosen T, de Wit PGJM, van den Broek HWJ (1993) Disruption of the avirulence gene avr9 in two races of the tomato pathogen Cladosporium fulvum causes virulence on tomato genotypes with the complementary resistance gene Cf9. Mol Plant-Microbe Interact 6:412–417
Miller BL, Miller KY, Timberlake WE (1985) Direct and indirect gene replacement in Aspergillus nidulans. Mol Cell Biol 5:1714–1721
Paietta JV, Marzluf GA (1985) Gene disruption by transformation in Neurospora crassa. Mol Cell Biol 5:1554–1559
Panaccione DG, Scott-Craig JS, Pocard J-A, Walton JD (1992) A cyclic peptide synthetase gene required for pathogenicity of the fungus Cochliobolus carbonum on maize. Proc Natl Acad Sci 89:6590–6594
Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, van den Hondel CAMJJ (1987) Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117–124
Raeder U, Broda P (1985) A rapid method for the purification of fungal DNA. Lett Appl Microbiol 1:17–20
Ried JL, Collmer A (1988) Construction and characterization of an Erwinia chrysanthemi mutant with directed deletions in all of the pectate lyase structural genes. Mol Plant-Microbe Interact 1:32–38
Rikkerink EHA, Solon SL, Crowhurst RN, Templeton MD (1994) Integration of vectors by homologous recombination in the plant pathogen Glomerella cingulata. Curr Genet 25:202–208
Rothstein RJ (1983) One-step gene disruption in yeast. Methods Enzymol 101:202–211
Sambrook J, Frisch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Scott-Craig JS, Panaccione DG, Cervone F, Walton JD (1990) Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell 2:1191–1200
Shortle D, Haber JE, Botstein D (1982) Lethal disruption of the yeast actin gene by integrative DNA transformation. Science 217:371–373
Skipp RA, Beever RE, Sharrock KR, Rikkerink EHA, Templeton MD (1994) Colletotrichum. In: Singh US, Kohmoto K, Singh RP (eds) Pathogenesis and host parasite specificity in plant disease: histopathological, genetic, biochemical and molecular basis. Elsevier Science Ltd., Oxford, UK (in press)
Stahl DJ, Schäfer W (1992) Cutinase is not required for fungal pathogenicity on pea. Plant Cell 4:621–629
Stotz HU, Powell ALT, Damon SE, Greve LC, Bennett AB, Labavitch JM (1993) Molecular characterization of a polygalacturonase inhibitor from Pyrus communis L. cv. Bartlett. Plant Physiol 102:133–138
Sutton TB (1990) Bitter rot. In: Jones AL, Aldwinkle HS (eds.) Compendium of apple and pear diseases. American Phytopathological Society Press, pp 15–16
Sweigard JA, Chumley FG, Valent B (1992) Disruption of a Magnaporthe grisea cutinase gene. Mol Gen Genet 232:183–190
Talbot NJ, Ebbole DJ, Hamer JE (1993) Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell 5:1575–1590
Templeton MD, Rikkerink EHA, Solon SL, Crowhurst RN (1992) Cloning and molecular characterization of the glyceraldehyde 3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 122:225–230
Templeton MD, Sharrock KR, Bowen JK, Crowhurst RN, Rikkerink EHA (1994) The pectin lyase gene family fron Glomerella cingulata: characterization of pnlA and its expression in yeast. Gene 142:141–146
Toubart P, Desiderio A, Salvi G, Cervone F, Daroda L, De Lorenzo G (1992) Cloning and characterization of the gene encoding the endopolygalacturonase-inhibiting protein (PGIP) of Phaseolus vulgaris. Plant Journal 2:367–373
Voelkel-Meiman K, Keil RL, Roeder GS (1987) Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell 48:1071–1079
Vogel HJ (1964) Distribution of lysine pathways among fungi: evolutionary implications. Amer Naturalist 98:435–446
Author information
Authors and Affiliations
Additional information
Communicated by C. A. M. J. J. van den Hondel
Rights and permissions
About this article
Cite this article
Bowen, J.K., Templeton, M.D., Sharrock, K.R. et al. Gene inactivation in the plant pathogen Glomerella cingulata: three strategies for the disruption of the pectin lyase gene pnIA . Molec. Gen. Genet. 246, 196–205 (1995). https://doi.org/10.1007/BF00294682
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00294682