Summary
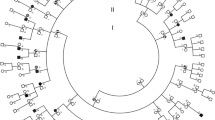
Leukocyte mitochondrial DNA (mtDNA) from 17 Finnish families iwth Leber's hereditary optic neuroretinopathy and 70 maternally unrelated controls as well as skeletal muscle mtDNA from four of the Leber families and three controls was analyzed with 30 restriction enzymes. By this means, over 10% of the nucleotides of mtDNA were screened. No major deletion or insertion was found in any of the mtDNAs studied. The restriction fragment patterns of mtDNA showed no evidence of mtDNA heteroplasmy (mixture of different mtDNA types) in either blood or muscle cells. In all, 24 mtDNA types were observed in the material. In the maternal lines of Leber families, 11 mtDNA types were found, indicating no recent common maternal ancestor for the Finnish Leber families. In spite of several previously unknown polymorphisms, no mutation of mtDNA could be found exclusively in families with Leber's disease. However, a couple of mutations leading to amino acid replacements of mitochondrially encoded proteins were observed in certain Leber families only. These mutations have occurred in genes coding for subunits of NADH dehydrogenase, suggesting that a defect of the respiratory chain complex I may cause Leber's disease.
Similar content being viewed by others
References
Anderson S, Bankier AT, Barrell BG, deBrujin MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJH, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290:457–465
Cann RL, Brown WM, Wilson AC (1984) Polymorphic sites and the mechanisms of evolution in human mitochondrial DNA. Genetics 106:479–499
Cann RL, Stoneking M, Wilson AC (1987) Mitochondrial DNA and human evolution. Nature 325:31–36
Chomyn A, Mariottini P, Cleeter MWJ, Ragan CI, Matsuno-Yagi A, Hatefi Y, Doolittle RF, Attardi G (1985a) Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature 314:592–597
Chomyn A, Mariottini P, Cleeter MWJ, Ragan CI, Matsuno-Yagi A, Hatefi Y, Doolittle RF, Attardi G (1985b) Functional assignment of the products of the unidentified reading frames of human mitochondrial DNA. In: Quagliarello E, Slater EC, Palmieri F, Saccone C, Kroon AM (eds) Achievements and perspectives in mitochondrial research. (Biogenesis, vol 2) Elsevier, New York Amsterdam, pp 259–276
DiMauro S, Bonilla E, Zeviani M, Nakagawa M, DeVivo DC (1985) Mitochondrial myopathies. Ann Neurol 17:521–535
Hauswirth WW, Laipis PJ (1985) Transmission genetics of mammalian mitochondria: a molecular model and experimental evidence. In: Quagliarello E, Slater EC, Palmieri F, Saccone C, Kroon AM (eds) Achievements and perspectives in mitochondrial research. (Biogenesis, vol 2) Elsevier, New York Amsterdam, pp 49–60
Holt IJ, Harding AE, Morgan-Hughes JA (1988) Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 331:717–719
Horai S, Gojobori T, Matsunaga E (1984) Mitochondrial DNA polymorphism in Japanese. I. Analysis with restriction enzymes of six base pair recognition. Hum Genet 68:324–332
Johnson MJ, Wallace DC, Gerris SD, Rattazzi MC, Cavalli-Sforza LL (1983) Radiation of human mitochondrial DNA types analyzed by restriction endonuclease cleavage patterns. J Mol Evol 19:255–271
Nikoskelainen E (1985) The clinical findings in Leber's hereditary optic neuroretinopathy. Trans Ophthalmol Soc UK 104:845–852
Nikoskelainen E, Hassinen IE, Paljärvi L, Lang H, Kalimo H (1984) Leber's hereditary optic neuroretinopathy, a mitochondrial disease? Lancet II:1474
Nikoskelainen EK, Savontaus M-L, Wanne OP, Katila MJ, Nummelin KU (1987) Leber's hereditary optic neuroretinopathy, a maternally inherited disease. A genealogic study in four pedigrees. Arch Ophthalmol 105:665–671
Seedorff T (1985) The inheritance of Leber's disease: a genealogical follow-up study. Acta Ophthalmol 63:135–145
Senus AHC van (1963) Leber's disease in the Netherlands. Doc Ophthalmol 17:1–162
Uemura A, Osame M, Nakagawa M, Nakahara K, Sameshima M, Ohba N (1987) Leber's hereditary optic neuropathy: mitochondrial and biochemical studies on muscle biopsies. Br J Ophthalmol 71:531–536
Vilkki J, Savontaus M-L, Nikoskelainen EK (1988) Human mitochondrial DNA types in Finland. Hum Genet 80:317–321
Wallace DC (1970) Leber's optic atrophy: a possible example of vertical transmission of a slow virus in man. Aust Ann Med 19:1–4
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vilkki, J., Savontaus, ML., Kalinmo, H. et al. Mitochondrial DNA polymorphism in Finnish families with Leber's hereditary optic neuroretinopathy. Hum Genet 82, 208–212 (1989). https://doi.org/10.1007/BF00291155
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00291155