Abstract
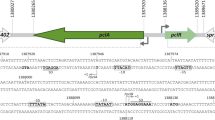
On the Streptococcus equisimilis H46A chromosome, the divergent coding sequences of the genes for the plasminogen activator streptokinase (skc) and a leucine-rich protein (lrp), the function of which is unknown, are separated by a 328 by intrinsically bent DNA region rich in AT tracts. To begin to understand the expression control of these two genes, we mapped their transcriptional initiation sites by S1 nuclease analysis and studied the influence of the bent intergenic region on promoter strength, using promoter-reporter gene fusions of skc′ and lrp′ to ′lacZ from Escherichia coli. The major transcriptional start sites, in both S. equisimilis and E. coli, mapped 22 bases upstream of the ATG start site of lrp (G), and 24 and 32 bases upstream of the translational initiation codon of skc (A and G, respectively), indicating the existence of two overlapping canonical skc promoters arranged in tandem on opposite faces of the helix. The reporter gene fusions were cloned in E. coli on a vector containing a 1.1 kb fragment of the S. equisimilis dexB gene, thus allowing promoter strength to be measured in multiple plasmid-form copies in the heterologous host and in single-copy genomic form following integration into the skc region of the homologous host. In S. equisimilis, skc′-′lacZ was expressed about 200-fold more strongly than the corresponding lrp′-′lacZ fusion. In contrast, in E. coli, the corresponding levels of expression differed by only about 11-fold. Deletion of the 202 by bent region upstream of the skc and lrp core promoters caused a 13-fold decrease in skc promoter activity in S. equisimilis but did not alter lrp promoter strength in this host. In contrast, when studied in E. coli, this deletion did not alter the strength of the skc double promoter and even increased by 2.4- to 3-fold the activity of the lrp promoter. This comparative promoter analysis shows that skc has a complex promoter structure, the activity of which in the homologous genomic environment specifically depends on sequences upstream of the two core promoters. Thus, the skc promoter structure resembles that of an array of promoters involved in a transcriptional switch; however, the nature of the potential switch factor(s) remains unknown.
Similar content being viewed by others
References
Barthelemy I, Salas M, Mellado RP (1987) In vivo transcription of bacteriophage θ29 DNA transcription initiation sites. J Virol 60:874–879
Berge A, Sjöbring U (1993) PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J Biol Chem 268:25417–25424
Brune B, Dimmeler S, Vedia LMY, Lapetina EG (1994) Nitric oxide: a signal for ADP-ribosylation of proteins. Life Sci 54:61–70
Caparon MG, Scott JR (1991) Genetic manipulation of pathogenic streptococci. Methods Enzymol 204:556–586
Carter P, Bedouelle U, Winter G (1985) Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res 13:4431–4443
Christensen LR (1945) Streptococcal fibrinolysin: a proteolytic reaction due to a serum enzyme activated by streptococcal fibrinolysin. J Gen Physiol 28:363–383
Collado-Vides J, Magasanik B, Gralla JD (1991) Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev 55:371–394
de Lorenzo V, Herrero M, Giovanni F, Neilands JB (1988) Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem 173:537–546
Deuschle U, Kammerer W, Gentz R, Bujard H (1986) Promoters of Escherichia coli: a hierarchy of in vivo strength indicates alternate structures. EMBO J 5:2987–2994
Deutsch DG, Mertz ET (1970) Plasminogen purification from human plasma. Science 170:1095–1096
Ellinger T, Behnke D, Knaus R, Bujard H, Gralla JD (1994a) Context-dependent effects of upstream A-tracts: stimulation or inhibition of Escherichia coli promoter function. J Mol Biol 239:466–475
Ellinger T, Behnke D, Bujard H, Gralla JD (1994b) Stalling of Escherichia coli RNA polymerase in the + 6 to + 12 region in vivo is associated with tight binding to consensus promoter elements. J Mol Biol 239:455–465
Gartenberg MR, Crothers DM (1991) Synthetic DNA bending sequences increase the rate of in vitro transcription initiation at the Escherichia coli lac promoter. J Mol Biol 219:217–230
Gentz R, Bujard H (1985) Promoters recognized by E. coli RNA polymerase selected by function: highly efficient promoters from bacteriophage T5. J Bacteriol 164:70–77
GUSTO Angiographic Investigators (1993) The effects of tissue plasminogen activator, streptokinase, or both on coronaryartery patency, ventricular function, and survival after acute myocardial infarction. New Engl J Med 329:1615–1622
Knaus R, Bujard H (1988) PL of coliphage lambda: an alternative solution for an efficient promoter. EMBO J 7:2919–2923
Kuusela P, Ullberg M, Saksela O, Kronvall G (1992) Tissue-type plasminogen activator-mediated activation of plasminogen on the surface of group A, C, and G streptococci. Infect Immun 60:196–201
Lamond AI, Travers AA (1985a) Genetically separable functional elements mediate the optimal expression and stringent regulation of a bacterial tRNA gene. Cell 40:319–326
Lamond AI, Travers AA (1985b) Stringent control of bacterial transcription. Cell 41:6–8
Lottenberg RZ, Broder CC, Boyle MPD, Kain SJ, Schroeder BL, Curtiss III R (1992a) Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J Bacteriol 174:5204–5210
Lottenberg R, DesJardin LE, Wang H, Boyle MDP (1992b) Streptokinase-producing streptococci grown in human plasma acquire unregulated cell-associated plasmin activity. J Infect Dis 166:436–440
Malke H, Roe B, Ferretti JJ (1985) Nucleotide sequence of the streptokinase gene from Streptococcus equisimilis H46A. Gene 34:357–362
Malke H, Mechold U, Gase K, Gerlach D (1994) Inactivation of the streptokinase gene prevents Streptococcus equisimilis H46A from acquiring cell-associated plasmin activity in the presence of plasminogen. FEMS Microbiol Lett 116:107–112
McAllister CF, Achberger EC (1988) Effect of polyadenine-containing curved DNA on promoter utilization in Bacillus subtilis. J Biol Chem 263:11743–11749
McAllister CF, Achberger EC (1989) Rotational orientation of upstream curved DNA affects promoter function in Bacillus subtilis. J Biol Chem 264:10451–10456
McClure WR (1985) Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem 54:171–204
Mechold U, Steiner K, Vettermann S, Malke H (1993) Genetic organization of the streptokinase region of the Streptococcus equisimilis H46A chromosome. Mol Gen Genet 241:129–140
Mechold U, Cashel M, Steiner K, Gentry D, Malke H (1994) Functional analysis of the rel gene of Streptococcus equisimilis. 4th International ASM Conference on Streptococcal Genetics, Santa Fe, NM; Abstract T8
Miller JH (1992) A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 72–74
Müller J, Malke H (1989) Sequence-directed DNA bending upstream of the streptokinase promoter. J Basic Microbiol 29:611–616
Müller J, Reinert H, Malke H (1989) Streptokinase mutations relieving Escherichia coli K12 (prlA4) of detriments caused by the wild-type skc gene. J Bacteriol 171:2202–2208
Ohlsen KL, Gralla JD (1992a) Melting during steady-state transcription of the rrnB P1 promoter in vivo and in vitro. J Bacteriol 174:6071–6075
Ohlsen KL, Gralla JD (1992b) Interrelated effects of DNA supercoiling, ppGpp, and low salt on melting within the Escherichia coli ribosomal RNA rrnB PI promoter. Mol Microbiol 6:2243–2251
Ohlsen KL, Gralla JD (1992c) DNA melting within stable closed complexes at the E. coli rrnB P1 promoter. J Biol Chem 267:19813–19818
Pagel JM, Winkelman JW, Adams CW, Hatfield GW (1992) DNA topology-mediated regulation of transcription initiation from tandem promoters of the ilvGMEDA operon of Escherichia coli. J Mol Biol 224:919–935
Pancholi V, Fischetti VA (1993) Glyceraldehyde-3-phosphate dehydrogenase on the surface of group A streptococci is also an ADP-ribosylating enzyme. Proc Natl Acad Sci USA 90:8154–8158
Pérez-Martín J, Rojo F, de Lorenzo V (1994) Promoters responsive to DNA bending: a common theme in prokaryotic gene expression. Microbiol Rev 58:268–290
Plaskon RR, Wartell RM (1987) Sequence distributions associated with DNA curvature are found upstream of strong E. coli promoters. Nucleic Acids Res 15:785–796
Rao L, Ross W, Appleman JA, Gaal T, Leirmo S, Schlax PJ, Record MT, Gourse RL (1994) Factor-independent activation of rrnB P1: an “extended” promoter with an upstream element that dramatically increases promoter strength. J Mol Biol 235:1421–1435
Reznikoff WS, Bertrand K, Donnelly C, Krebs M, Maquat LE, Peterson M, Wray L, Yin J, Yu X-M (1987) Complex promoters. In: Reznikoff WS, Gross CA, Burgess RR, Record MT, Dahlberg JE, Wickens MP (eds) RNA polymerase and the regulation of transcription. Elsevier Science Publishing, New York, pp 105–113
Ross W, Gosink KK, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse RL (1993) A third recognition element in bacterial promoters: DNA binding by the α subunit of RNA polymerase. Science 262:1407–1413
Sambrook J, Fritsch EF, Maniatis TE (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Silhavy TJ, Beckwith JR (1985) Uses of lac fusions for the study of biological problems. Microbiol Rev 49:398–418
Spassky A, Busby S, Buc H (1984) On the action of cyclic AMP-cyclic AMP receptor protein complex at the Escherichia coli lactose and galactose promoter regions. EMBO J 3:43–50
Steiner K, Malke H (1995) Transcription termination of the streptokinase gene of Streptococcus equisimilis H46A: bidirectionality and efficiency in homologous and heterologous hosts. Mol Gen Genet 246:374–380
Travers AA, Lamond AI, Mace HAF, Berman ML (1983) RNA polymerase interactions with upstream region of the E. coli tyrT promoter. Cell 35:265–273
Wang J-Y, Syvanen M (1992) DNA twist as a transcriptional sensor for environmental changes. Mol Microbiol 6:1861–1866
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mpl8 and pUC19 vectors. Gene 33:103–119
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gase, K., Ellinger, T. & Malke, H. Complex transcriptional control of the streptokinase gene of Streptococcus equisimilis H46A. Molec. Gen. Genet. 247, 749–758 (1995). https://doi.org/10.1007/BF00290407
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00290407