Abstract
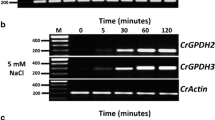
Yeast cells can respond and adapt to osmotic stress. In our attempt to clarify the molecular mechanisms of cellular responses to osmotic stress, we cloned seven cDNAs for hyperosmolarity-responsive (HOR) genes from Saccharomyces cerevisiae by a differential screening method. Structural analysis of the clones revealed that those designated HOR1, HORS, HOR4, HOR5 and HOR6 encoded glycerol-3-phosphate dehydrogenase (Gpd1p), glucokinase (Glklp), hexose transporter (Hxtlp), heat-shock protein 12 (Hsp12p) and Na+, K+, Li+-ATPase (Enalp), respectively. HOR2 and HOR7 corresponded to novel genes. Gpdlp is a key enzyme in the synthesis of glycerol, which is a major osmoprotectant in S. cerevisiae. Cloning of HOR1/GPD1 as a HOR gene indicates that the accumulation of glycerol in yeast cells under hyperosmotic stress is, at least in part, caused by an increase in the level of GPDH protein. We performed a series of Northern blot analyses using HOR cDNAs as probes and RNAs prepared from cells grown under various conditions and from various mutant cells. The results suggested that all the HOR genes are regulated by common signal transduction pathways. However, the fact that they exhibited certain distinct responses indicated that they might also be regulated by specific pathways in addition to the common pathways. Ca2+ seemed to be involved in the signaling systems. In addition, Hog1p, one of the MAP kinases in yeast, appeared to be involved in the regulation of expression of HOR genes, although its function seemed to be insufficient for the overall regulation of expression of these genes.
Similar content being viewed by others
References
Albertyn J, Hohmann S, Thevelein JM, Prior BA (1994) GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol Cell Biol 14:4135–4144
Albig W, Entain K-D (1988) Structure of yeast glucokinase, a strongly diverged specific aldo-hexose-phosphorylating isoenzyme. Gene 73:141–152
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1987) Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York
Bisson LF, Fraenkel DG (1983) Involvement of kinase in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci USA 80:1730–1734
Bray EA (1993) Molecular responses to water deficit. Plant Physiol 103:1035–1040
Brewster JL, Valoir TD, Dwyer ND, Winter E, Gustin MC (1993) An osmosensing signal transduction pathway in yeast. Science 259:1760–1763
Brown AD (1978) Compatible solutes and extreme water stress in eukaryotic micro-organism. Adv Microbial Physiol 17: 181–242
Claes B, Dekeyser R, Villarroel R, Bulcke MVD, Bauw G, Montagu MV, Caplan A (1990) Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell 2:19–27
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant 14: 215–223
Eraso P, Cid A, Serrano R (1987) Tight control of the amount of yeast plasma membrane ATPase during changes in growth conditions and gene dosage. FEBS Lett 224:193–197
Garciadeblas B, Rubio F, Quintero FJ, Bañuelos MA, Haro R, Rodríguez-Navarro A (1993) Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol Gen Genet 236:363–368
Gaxiola R, Larrinoa IFD, Villalba JM, Serrano R (1992) A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO J 11:3157–3164
Gietz RD, Sugino A (1988) New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527–534
Gläser H-U, Thomas D, Gaxiola R, Montrichard F, Surdin-Kerjan Y, Serrano R (1993) Salt tolerance and methionine biosynthesis in Saccharomyces cerevisiae involve a putative phosphatase gene. EMBO J 12:3105–3110
Haro R, Garciadeblas B, Rodriguez-Navarro A (1991) A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett 291:189–191
Hendrick JP, Hard F-U (1993) Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem 62:349–384
Hirayama T, Oka A (1992) Novel protein kinase of Arabidopsis thaliana (APKI) that phosphorylates tyrosine, serine and threonine. Plant Mol Biol 20:653–662
Iida H, Yahara I (1985) Yeast heat shock protein of M{INR 48,000 is an isoprotein of enolase}. Nature 315:688–690
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K (1994) Cloning of cDNAs for genes that are early-responsive to dehydration stress (ERDs) in Arabidopsis thahana L.: identification of three ERDs as HSP cognate genes. Plant Mol Biol 25:791–798
Lee KS, Levin DE (1992) Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol Cell Biol 12:172–182
Levin DE, Bartlett-Heubusch E (1992) Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol 116:1221–1229
Levin DE, Fields FO, Kunisawa R, Bishop JM, Thorner J (1990) A. candidate protein kinase C gene; PKC1, is required for the S. cerevisiae cell cycle. Cell 62:213–224
Lewis DA, Bisson LF (1991) The HXT1 gene product of Saccharomyces cerevisiae is a new member of the family of hexose transporters. Mol Cell Biol 11:3804–3813
Maeda T, Wurgler-Murphy SM, Saito H (1994) A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369:242–245
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory press, Cold Spring Harbor, NY
Marchler G, Schüller C, Adam G, Ruis H (1993) A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J 12:1997–2003
Matsumoto K, Uno I, Oshima Y, Ishikawa T (1982) Isolation and characterization of yeast mutants deficient in adenylate cyclase and CAMP-dependent protein kinase. Proc Natl Acad Sci USA 79:2355–2359
McCue KF, Hanson AD (1992) Salt-inducible betaine aldehyde dehydrogenase from sugar beet: cDNA cloning and expression. Plant Mol Biol 18:1–11
Nakamura T, Liu Y, Hirata D, Namba H, Harada S-I, Hirokawa T, Miyakawa T (1993) Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J 12:4063–4071
Piatkowski D, Schneider K, Salamini F, Bartels D (1990) Characterization of five abscisic acid-responsive cDNA clones isolated from the desiccation-tolerant plant Craterostigma plantagineum and their relationship to other water-stress genes. Plant Physiol 94:1692–1698
Piper P, Curran B, Davies M, Hirst K, Lockheart A, Ogden J, Stanway C, Kingsman A, Kingsman S (1988) A heat shock element in the phosphoglycerate kinase gene promoter of yeast. Nucleic Acids Res 16:1333–1348
Praekelt UM, Meacock PA (1990) HSP12, a new small heat shock gene of Saccharomyces cerevisiae: Analysis of structure, regulation and function. Mol Gen Genet 223:97–106
Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, Davidow LS, Mao J-I, Moir DT (1989) The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58:133–145
Schüller C, Brewster JL, Alexander MR, Gustin MC, Ruis H (1994) The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J 13:4382–4389
Sherman F (1991) Getting start with yeast. In: Guthrie C, Fink GR (eds) Guide to yeast genetics and molecular biology. Academic Press, San Diego, pp 3–21
Shimizu J, Yoda K, Yamasaki M (1994) The hypo-osmolarity-sensitive phenotype of the Saccharomyces cerevisiae hop2 mutant is due to a mutation in PKC1, which regulates expression of β-glucanase. Mol Gen Genet 242:641–648
Sikorski RJ, Heiter P (1989) A system of shuttle vectors and host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 112:19–27
Sleep D, Ogden JE, Roberts NA, Goodey AR (1991) Cloning and characterization of the Saccharomyces cerevisiae glycerol-3-phosphate dehydrogenase (GUT2) promoter. Gene 101:89–96
Tanaka K, Matsumoto K, Toh-e A (1988) Dual regulation of the expression of the polyubiquitin gene by cyclic AMP and heat shock in yeast. EMBO J 7:495–502
Varela JCS, Beekvelt CV, Planta RJ, Mager WH (1992) Osmostress-induced changes in yeast gene expression. Mol Microbiol 6:2183–2190
Werner-Washburne M, Becker J, Kosic-Smithers J, Carig EA (1989) Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol 171:2680–2688
Yamaguchi-Shinozaki K, Koizumi M, Urao S, Shinozaki K (1992) Molecular cloning and characterization of 9 cDNAs for genes that are responsive to desiccation in Arabidopsis thaliana; Sequence analysis of one cDNA clone that encodes a putative transmembrane channel protein. Plant Cell Physiol 33:217–224
Author information
Authors and Affiliations
Additional information
Communicated by M. Sekiguchi
Rights and permissions
About this article
Cite this article
Hirayarna, T., Maeda, T., Saito, H. et al. Cloning and characterization of seven cDNAs for hyperosmolarity-responsive (HOR) genes of Saccharomyces cerevisiae . Molec. Gen. Genet. 249, 127–138 (1995). https://doi.org/10.1007/BF00290358
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00290358