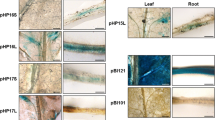
Abstract
The key enzymes of photosynthetic carbon assimilation in C4 plants have evolved from C3 isoforms which were present in the C3 ancestral species. We are interested in the molecular changes responsible for the novel expression pattern of C4 genes and are focussing on phosphoenolpyruvate carboxylase (PEPCase) of the genus Flaveria. The C4 isoform of PEPCase in the C4 plant F. trinervia is encoded by the ppcA subgroup of the PEPCase gene family and is abundantly expressed in the mesophyll cells of leaves. The orthologous ppcA genes of the C3 plant F. pringlei are only weakly expressed and their transcripts do not accumulate in a leaf-specific manner but, rather, are present in all plant organs. To answer the question whether the differences in the expression levels of the ppcA genes from F. pringlei and F. trinervia are caused by changes in the 5′ upstream regions of the genes or by C4-specific trans-regulatory factors, varying parts of the 5′ flanking region of the ppcA1 genes of both species were fused to the β-glucuronidase (GUS) gene and inserted in the tobacco genome. GUS expression analysis of transgenic plants revealed that the level of expression of the Flaveria ppcA1 genes are recapitulated in the heterologous C3 plant tobacco. Hence, the 5′ upstream region of the ppcA1 gene of F. trinervia contains regulatory cis-elements that are responsible for the C4-specific, abundant expression of this gene. These sequences are located upstream of position −500 relative to the transcription initiation site. Interestingly, the ppcA1 promoter (position −2118 to position + 66) of F. trinervia is specifically active in palisade parenchyma cells of transgenic tobacco, suggesting that the mesophyll specificity of this promoter is partially maintained in tobacco.
Similar content being viewed by others
References
Bauwe H, Chollet R (1986) Kinetic properties of phosphoenolpyruvate carboxylase from C3, C4, and C3-C4 intermediate species of Flaveria (Asteraceae). Plant Physiol 82:695–699
Benfey PN, Chua NH (1989) Regulated genes in transgenic plants. Science 244:174–181
Benfey PN, Ren L, Chua NH (1989) The CaMV 35S enhancer contains at least two domains which can confer different developmental and tissue-specific expression patterns. EMBO J 8:2195–2202
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown RH, Hattersley PW (1989) Leaf anatomy of C3-C4 species as related to evolution of C4 photosynthesis. Plant Physiol 91:1543–1550
Crétin C, Santi S, Keryer E, Lepiniec L, Tagu D, Vidal J, Gadal P (1991) The phosphoenolpyruvate carboxylase gene family of Sorghum: promoter structures, amino acid sequences and expression of genes. Gene 99:87–94
Dean C, Jones J, Favreau M, Dunsmuir P, Bedbrook J (1988) Influence of flanking sequences on variability in expression levels of an introduced gene in transgenic tobacco plants. Nucleic Acids Res 16:9267–9283
Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, van Montagu M, Leemans J (1985) Efficient octopine Ti plasmidderived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res 13:4777–4788
Harpster MH, Taylor WC (1986) Maize phosphoenolpyruvate carboxylase. Cloning and characterization of mRNAs encoding isozymic forms. J Biol Chem 261:6132–6136
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895:81–106
Hermans J, Westhoff P (1990) Analysis of expression and evolutionary relationships of phosphoenolpyruvate carboxylase genes in Flaveria trinervia (C4) and F. pringlei (C3). Mol Gen Genet 224:459–468
Hermans J, Westhoff P (1992) Homologous genes for the C4 isoform of phosphoenolpyruvate carboxylase in a C3−C4and a C4 Flaveria species. Mol Gen Genet 234:275–284
Hobbs SLA, Kpodar P, DeLong CMO (1990) The effect of TDNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol 15:851–864
Horsch RB, Fry FE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Hudspeth RL, Grula JW, Dai Z, Edwards GE, Ku MSB (1992) Expression of maize phosphoenolpyruvate carboxylase in transgenic tobacco. Effects on biochemistry and physiology. Plant Physiol 98:458–464
Höfer MU, Santore UJ, Westhoff P (1992) Differential accumulation of the 10-, 16- and 23-kDa peripheral components of the water-splitting complex of photosystem 11 in mesophyll and bundle-sheath chloroplasts of the dicotyledonous C4 plant Flaveria trinervia (Spring.) C. Mohr. Planta 186:304–312
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kawamura T, Shigesada K, Toh H, Okumura S, Yanagisawa S, Izui K (1992) Molecular evolution of phosphoenolpyruvate carboxylase for C4 photosynthesis in maize: comparison of its cDNA sequence with a newly isolated cDNA encoding an isozyme involved in anaplerotic function. J Biochem 112:147–154
Kosugi S, Ohashi Y, Nakajima K, Arai Y (1990) An improved assay for β-glucuronidase in transformed cells: methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant Sci 70:133–140
Latzko E, Kelly J (1983) The multi-faceted function of phosphoenolpyruvate carboxylase in C3 plants. Physiol Vég 21:805–815
Lepiniec L, Keryer E, Philippe H, Gadal P, Cretin C (1993) Sorghum phosphoenolpyruvate carboxylase gene family: structure, function and molecular evolution. Plant Mol Biol 21:487–502
Matsuoka M, Sanada Y (1991) Expression of photosynthetic genes from the C4 plant, maize, in tobacco. Mol Gen Genet 225:411–419
Matsuoka M, Tada Y, Fujimura T, Kano-Murakami Y (1993) Tissue-specific light-regulated expression directed by the promoter of a C4 gene, maize pyruvate, orthophosphate dikinase, in a C3 plant, rice. Proc Natl Acad Sci USA 90:9586–9590
Monson RK, Moore BD (1989) On the significance of C3-C4 intermediate photosynthesis to the evolution of C4 photosynthesis. Plant Cell Environ 12:689–699
Moore PD (1982) Evolution of photosynthetic pathways in flowering plants. Nature 295:647–648
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Poetsch W, Hermans J, Westhoff P (1991) Multiple cDNAs of phosphoenolpyruvate carboxylase in the C4 dicot Flaveria trinervia. FEBS Lett 292:133–136
Powell AM (1978) Systematics of Flaveria (Flaveriinae-Asteraceae). Ann Missouri Bot Gard 65:590–636
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Stockhaus J, Eckes P, Rocha-Sosa M, Schell J, Willmitzer L (1987) Analysis of cis-active sequences involved in the leaf-specific expression of a potato gene in transgenic plants. Proc Natl Acad Sci USA 84:7943–7947
Stockhaus J, Schell J, Willmitzer L (1989) Correlation of the expression of the nuclear photosynthetic gene ST-LS1 with the presence of chloroplasts. EMBO J 8:2445–2451
Tagu D, Cretin C, Bergounioux C, Lepiniec L, Gadal P (1991) Transcription of a sorghum phosphoenolpyruvate carboxylase gene in transgenic tobacco leaves: maturation of monocot pre-mRNA by dicot cells. Plant Cell Rep 9:688–690
Weising K, Schell J, Kahl G (1988) Foreign genes in plants: transfer, structure, expression, and applications. Annu Rev Genet 22:421–177
Wilson C, Bellen HJ, Gehring WJ (1990) Position effects on eukaryotic gene expression. Annu Rev Cell Biol 6:679–714
Author information
Authors and Affiliations
Additional information
Communicated by R. G. Herrmann
Rights and permissions
About this article
Cite this article
Stockhaus, J., Poetsch, W., Steinmüller, K. et al. Evolution of the C4 phosphoenolpyruvate carboxylase promoter of the C4 dicot Flaveria trinervia: an expression analysis in the C3 plant tobacco. Molec. Gen. Genet. 245, 286–293 (1994). https://doi.org/10.1007/BF00290108
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00290108