Summary
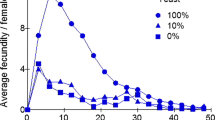
The relationship between heterozygosity and the expression of heterosis at two different nutrition levels was investigated using Drosophila melanogaster. Average daily egg production and egg hatchability were measured in two parental strains and in F1, F2 and reciprocal backcross generations. Heterosis was more pronounced in the poor nutritional conditions. Two electrophoretic markers used to estimate the level of heterozygosity in F2 and backcrosses revealed an excess of heterozygous genotypes. Quantitative genetic effects (an additive line effect and individual and maternal heterosis) were estimated for both traits in the two environments. Although this model gave a reasonable fit in most cases, some epistatic interaction would have to be invoked in order to explain fully the results.
Similar content being viewed by others
References
Barlow R (1981) Experimental evidence for interaction between heterosis and environment in animals. Anim Breed Abstr 49:715–737
Bird SR, Semeonoff R (1982) Electrophoretic analysis of many enzyme loci from a single fly homogenate. Dros Inf Ser 58:153
Boylan WJ (1982) Crossbreeding for fecundity. In: Coop IE (ed) Sheep and goat production. Elsevier, Amsterdam
Briscoe DA, Robertson A, Malpica J-M (1975) Dominance at Adh locus in response of adult Drosophila melanogaster to environmental alcohol. Nature 255:148–149
Cunningham EP (1985) Theoretical aspects of crossbreeding. FAO/UNEP Expert Consult Methodol Anim Genet Resources Data Banks (AGR/DB) Rome, June 1985
Cunningham EP (1987) Crossbreeding — the Greek-temple model. Z Tierz Züchtungsbiol 104:2–11
Falconer DS (1955) Pattern of response in selection experiments with mice. Cold Spring Harbor Symp Quant Biol 20:178–196
Falconer DS (1960) The genetics of litter size in mice. J Cell Comp Physiol 56:153–167
Falconer DS (1981) Introduction to quantitative genetics, 2nd ed. Longman, London
Festing MFW (1976) Effect of marginal malnutrition on the breeding performance of inbred and F1 hybrid mice — a diallel study. In: The laboratory animal in the study of reproduction. G. Fischer, Stuttgart (6th Symp Inter Comm Lab Animals, Thessaloniki, July 1975, pp 99–113)
Gowen JW (1952) Hybrid vigor in Drosophila. In: Gowen JW (ed) Heterosis. Iowa State College Press, Ames/IA, pp 474–493
Hohenboken WD (1985) Phenotypic, genetic and environmental correlation and genotype × environment interaction. In: Chapman AB (ed) General and quantitative genetics. Elsevier, Amsterdam
Kidwell MG (1979) Hybrid dysgenesis in Drosophila melanogaster: the relationship between the P-M and I-R interaction systems. Genet Res 33:205–217
Klosterman EW, Cahill VR, Parker CF (1968) A comparison of the Hereford and Charolais breeds and their crosses under two systems of management. Res Bull, Ohio Agr Res Dev Centre no 1011
Koch RM, Dickerson GE, Cundiff LV, Gregory KE (1985) Heterosis retained in advanced generations of crosses among Angus and Hereford cattle. J Anim Sci 60:1117–1132
McDaniel RG, Grimwood BC (1971) Hybrid vigour in Drosophila: Respiration and mitochondrial energy conservation. Comp Biochem Physiol 38:309–315
McWilliam JR, Griffing B (1965) Temperature-dependent heterosis in maize. Aust J Biol Sci 18:569–583
Oltenacu EAB, Boylan WJ (1982) Productivity of purebred and crossbred Finnsheep II. Lamb weight and production indices of ewes. J Anim Sci 52:998–1006
Pederson DG (1968) Environmental stress, heterozygote advantage and genotype-environment interaction in Arabidopsis. Heredity 23:127–138
Reily JG, Thomas CA (1980) Length polymorphisms, restriction site variation and maternal inheritance of mitochondrial DNA of Drosophila melanogaster. Plasmid 3:109–115
Rich SS, Bell AE (1980) Genotype-environment interaction effects in long-term selected populations of Tribolium. J Heredity 71:319–322
Robertson FW, Reeve ECR (1955) Studies in quantitative inheritance VIII. Further analysis of heterosis in crosses between inbred lines of Drosophila melanogaster. Z Indukt Abstamm Vererbungsl 86:439–458
Sang JH (1964) Nutritional requirements of inbred lines and crosses of Drosophila melanogaster. Genet Res 5:50–67
Sharp PM (1984) The effect of inbreeding on competitive malemating ability in Drosophila melanogaster. Genetics 106:601–612
Sheridan AK (1980) A new explanation for egg production heterosis in crosses between White Leghorns and Australorps. Brit Poultry Sci 21:85–88
Sheridan AK (1981) Crossbreeding and heterosis. Anim Breed Abstr 49:131–144
Sved JA (1971) An estimate of heterosis in Drosophila melanogaster. Genet Res 18:97–105
Sved JA (1975) Fitness of third chromosome homozygotes in Drosophila melanogaster. Genet Res 25:197–200
Syrstad O (1985) Heterosis in Bos taurus × Bos indicus crosses. Livest Prod Sci 12:299–307
Van der Steen HAM, Groot PND (1984) Genotype-energy intake interaction for growth and backfat thickness in gilts. 35th EAAP Meet, The Hague, August 6–9, 1984
Vetukhiv M (1956) Fecundity of hybrids between geographical populations of Drosophila pseudoobscura. Evolution 10: 139–146
Weir BS, Avery PJ, Hill WJ (1980) Effect of mating structure on variation in inbreeding. Theor Popul Biol 18:396–429
Author information
Authors and Affiliations
Additional information
Communicated by L. D. Van Vleck
Rights and permissions
About this article
Cite this article
Ruban, P.S., Cunningham, E.P. & Sharp, P.M. Heterosis × nutrition interaction in Drosophila melanogaster . Theoret. Appl. Genetics 76, 136–142 (1988). https://doi.org/10.1007/BF00288844
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00288844