Abstract
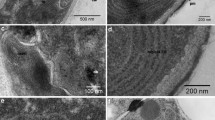
The intracellular location of the “low-molecular weight, alkaline” xylanase (XYN II) of Trichoderma reesei RUT C-30 was investigated during growth on xylan, using immunoelectron microscopy. A monoclonal antibody, produced against XYN II, was used for this purpose. The enzyme was found at the endoplasmic reticulum and in electron dense 0.2 to 0.8 μm vesicles, as well as in the vacuole, at the plasma membrane and in the fungal cell-wall. No staining occured in the cytoplasm, the mitochondria and the nucleus. No Golgi-like structures could be seen. Addition of the carboxylic ionophore monensin blocked xylanase as well as total protein secretion. The results are discussed with respect to XYN II being secreted by T. reesei via a pathway involving the endoplasmic reticulum and secretory vesicles and/or the vacuole.
Similar content being viewed by others
References
DargentR, Touze-SouletJM, RamiJ, MontantC (1982) Cytochemical characterization of Golgi apparatus in some filamentous fungi. Exp Mycol 6: 101–114
EnariTM, Niku-PaavolaML (1987) Enzymatic hydrolysis of cellulose: is the current theory of the mechanism of hydrolysis valid? CRC Crit Rev Biotechnol 5: 67–87
GhoseTK (1987) Cellulase activity determination. Pure Appl Chem 59: 257–268
GhoshA, Al-RabiaiS, GhoshBK, Trimino-VasquezH, EveleighDE, MontenecourtBS (1982) Increased endoplasmic reticulum content of a mutant of Trichoderma reesei (RUT C-30) in relation to cellulase synthesis. Enzyme Microb Technol 4: 110–113
GhoshA, GhoshBK, Trimino-VasquezH, EveleighDE, Montenecourt BS (1984) Cellulase secretion from a hyper-cellulolytic mutant of Trichoderma reesei RUT C-30. Arch Microbiol 140: 126–133
GhoshBK, TangulyT, GhoshA (1990) Ultrastructural aspects of cellulase biosynthesis and secretion. In: KubicekCP, EveleighDE, EsterbauerH, SteinerW, Kubicek-PranzEM (eds) Trichoderma reesei cellulases: biochemistry, physiology, genetics and applications. Royal Chemical Society Press, Cambridge, UK, pp 115–138
Humbel BM (1984) Gefriersubstitution — Ein Weg zur Verbesserung der morphologischen und cytochemischen Untersuchung biologischer Proben im Elektronenmikroskop. Dissertation, ETH Zürich
KuanIC, TienM (1989) Phosphorylation of lignin peroxidases from Phanerochaete chrysosporium. Identification of mannose-6-phosphate. J Biol Chem 264: 20350–20355
KubicekCP, PandaT, Schreferl-KunarG, GruberF, MessnerR (1987) O-linked but not N-linked glycosylation is necessary for the secretion of endoglucanases I and II by Trichoderma reesei. Can J Microbiol 33: 698–703
Kubicek CP (1992) The cellulase proteins of Trichoderma reesei: structure, multiplicity, mode of action and regulation of formation. Adv Biochem Engineer Biotechnol 45: 1–28
KurzątkowskiW, PalissaH, LiemptHvan, DöhrenHvan, KleinkaufH, WolfWP, KuryŀowiczW (1991) Localization of isopenicillin N synthase in Penicillium chrysogenum PQ 96. Appl Microbiol Biotechnol 35: 517–520
LappalainenA (1986) Purification and characterization of xylanolytic enzymes from Trichoderma reesei. Biotechnol Appl Biochem 8: 437–448
MandelsM, AndreottiRE (1978) The cellulose to cellulase fermentation. Proc Biochem 13: 6–13
MerivuoriH, MontenecourtBS, SandsJA (1987) Ethanol perturbs glycosylation and inhibits hypersecretion in Trichoderma reesei. Appl Envir Microbiol 53: 463–465
PandaT, GruberH, KubicekCP (1987) Stimulation of protein secretion in Trichoderma reesei by Tween surfactants is not correlated with changes in enzyme localization or membrane fatty acid composition. FEMS Microbiol Lett 41: 85–90
TartakoffAM (1983) Perturbation of vesicular traffic with the carboxylic ionophora monensin. Cell 32: 1026–1028
TenkanenM, PulsJ, PoutanenK (1992) Two major xylanases of Trichoderma reesei. Enzyme Microb Technol 14: 566–574
TörrönenA, AffenzellerK, HoferF, MyohanenTA, BlaasD, HarkkiA, KubicekCP (1992a) The major xylanase of Trichoderma reesei: purification, and production of monoclonal antibodies. In: VisserJ, BeldmanG, Kusters-van SomerenMA, VoragenAGJ (eds) Xylans and xylanases. Elsevier, Amsterdam London New York, pp 501–505
TörrönenA, MachRL, MessnerR, GonzalezR, KalkkinenN, HarkkiA, KubicekCP (1992b) The two major xylanases of Trichoderma reesei: characterization of both enzymes and genes. Bio/Technol 10: 1461–1465
WongKKY, SaddlerJN (1992) Trichoderma xylanases, their properties and application. In: VisserJ, BeldmanG, Kusters-van SomerenMA, VoragenAGJ (eds.) Xylans and xylanases. Elsevier, Amsterdam London New York, pp 171–186
YaguchiM, RoyC, UjiieM, WatsonDC, WararchukW (1992a) Amino acid sequence of the low molecular weight xylanase from Trichoderma viride. In: VisserJ, BeldmannG, Kuster-van SomerenMA, VoragenAGJ (eds) Xylans and xylanases. Elsevier, Amsterdam London New York, pp 149–155
YaguchiM, RoyC, WatsonDC, RollinF, TanLUL, SeniorDJ, SaddlerJN (1992b) The amino acid sequence of the 20 kDa xylanase from Trichoderma harzianum E58. In: VisserJ, BeldmanG, Kuster-van SomerenMA, VoragenAGJ (eds) Xylans and xylanases. Elsevier, Amsterdam London New York, pp 435–439
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kurzątkowski, W., Solecka, J., Filipek, J. et al. Ultrastructural localization of cellular compartments involved in secretion of the low molecular weight, alkaline xylanase by Trichoderma reesei . Arch. Microbiol. 159, 417–422 (1993). https://doi.org/10.1007/BF00288587
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00288587