Abstract
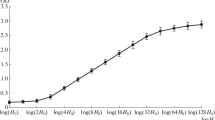
DNA topology in histone- and protamine-depleted nuclei (nucleoids) from somatic cells, sperm, and spermatogenic cells was studied to determine if the superhelical configuration of DNA looped domains is altered during spermatogenesis. The expansion and contraction of nucleoid DNA was measured with a fluorescence microscope following exposure of nucleoids to different concentrations of ethidium bromide (EB). Nucleoids from Xenopus laevis erythrocytes, primary spermatocytes, and round spermatids, and from Rana catesbeiana sperm all exhibited a biphasic change (condensed-relaxed-condensed) in size as a function of exposure to increasing concentrations (0.5–100 μg/ml) of EB, indicating that they contain negatively supercoiled DNA. In contrast, DNA in sperm nucleoids from Xenopus laevis and Bufo fowleri was relaxed and expanded at low (0.5–6 μg/ml) EB concentrations, but became gradually condensed as the EB concentration was increased (6–100 μg/ml). Nucleoids prepared from all cell types retained the general shape of the nucleus regardless of the superhelical configuration of the nucleoid DNA. Sperm nucleoid DNA condensed by 100 μg/ml EB was relaxed by exposure to UV light, DNase I, proteinase K, or 4 M urea, but not by RNase A or 10 mM dithiothreitol. These results demonstrate that the DNA in sperm nucleoids is constrained in domains of supercoiling by nonbasic nuclear proteins. Negatively supercoiled DNA is present in nucleoids from cells with a full complement of histones, including Rana sperm, but not in nucleoids from Xenopus and Bufo sperm in which histones are replaced by “intermediate-type” protamines. Histone replacement in these species, therefore, is accompanied by unfolding of nucleosomal DNA and active removal of the negative supercoils. Results presented also suggest an important role for the nonbasic nuclear proteins of sperm in the morphogenesis of the nucleus and the arrangement of DNA.
Similar content being viewed by others
References
Adolph KW, Cheng SM, Laemmli UH (1977) Role of non-histone proteins in metaphase chromosome structure, Cell 12:805–816
Adolph KW (1980) Organization of chromosomes in HeLa cells: Isolation of histone-depleted nuclei and nuclear scaffolds. J Cell Sci 42:291–304
Alder D, Gorovsky MA (1975) Electrophoretic analysis of liver and testis histones of the frog Rana pipiens. J Cell Biol 64:389–397
Ausio J, Subirana JA (1982) Nuclear proteins and organization of chromatin in spermatozoa of Mytilus edulis. Exp Cell Res 141:39–45
Balhorn R (1982) A model for the structure of chromatin in mammalian sperm. J Cell Biol 93:298–305
Bellvé AR, O'Brien DA (1983) The mammalian spermatozoon: Structure and temporal assembly. In: Hartmann JF (ed) Mechanism and control of animal fertilization. Academic Press, New York, pp 55–137
Benavente R, Krohne G (1985) Change of karyoskeleton during spermatogenesis of Xenopus: Expression of lamin LIV, a nuclear lamina protein specific for the male germ line. Proc Natl Acad Sci USA 82:6176–6180
Benyajati C, Worcel A (1976) Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell 9:393–407
Bloch DP (1976) Histones of sperm. In: King RC (ed) Handbook of genetics, vol 5. Plenum Press, New York, pp 139–167
Bode J, Willmitzer L, Opatz K (1977) On the competition between protamines and histones: studies directed towards the understanding of spermiogenesis. Eur J Biochem 72:393–403
Buongiorno-Nardelli M, Micheli G, Carrĩ MT, Marilley M (1982) A relationship between replicon size and supercoiled loop domains in the eukaryotic genome. Nature 298:100–102
Chevaillier PH (1983) Some aspects of chromatin organization in sperm nuclei. In: Andre J (ed) The sperm cell. Martinus Nijhoff, The Hague, pp 179–196
Cook PR, Brazell IA, Jost E (1976) Characterization of nuclear structures containing superhelical DNA. J Cell Sci 22:303–324
Cook PR, Brazell IA (1976) Conformational constraints in nuclear DNA. J Cell Sci 22:287–302
Cornudella L, Rocha E (1979) Nucleosome organization during germ cell development in the sea cucumber Holothuria tubulosa. Biochemistry 18:3724–3732
Easton D, Chalkley R (1972) High-resolution electrophoretic analysis of the histones from embryos and sperm of Arbacia punctulata. Exp Cell Res 72:502–508
Gusse M, Chevaillier PH (1980a) Molecular structure of chromatin during sperm differentiation of the dogfish Scyliorhinus caniculus (L.). Chromosoma 77:57–68
Gusse M, Chevaillier PH (1980b) Electron microscope evidence for the presence of globular structures in different sperm chromatins. J Cell Biol 87:280–284
Honda BM, Baillie DL, Candido EPM (1974) The subunit structure of chromatin: Characteristics of nucleohistone and nucleoprotamine from developing trout testis. FEBS Lett 48:156–159
Igó-Kemenes T, Zachau HG (1978) Domains in chromatin structure. Cold Spring Harbor Symp Quant Biol 42:109–118
Kasinsky HE, Huang SY, Mann M, Roca J, Subirana JA (1985) On the diversity of sperm histones in the vertebrates: IV. Cytochemical and amino acid analysis in Anura. J Exp Zool 234:33–46
Kaufman SH, Shaper JH (1984) A subset of non-histone nuclear proteins reversibly stabilized by the sulfhydral cross-linking reagent tetrathionate. Exp Cell Res 155:477–495
Kierszenbaum AL, Tres LL (1975) Structural and transcriptional features of the mouse spermatid genome. J Cell Biol 65:258–270
Kierszenbaum AL, Tres LL (1978) The packing unit: A basic structural feature for the condensation of late cricket spermatid nuclei. J Cell Sci 33:265–283
Kunkle M (1984) Structural components of sea urchin sperm nuclei. Gamete Res 8:357–370
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lebkowski JS, Laemmli UK (1982a) Evidence for two levels of DNA folding in histone-depleted Hela interphase nuclei. J Mol Biol 156:309–324
Lebkowski JS, Laemmli UK (1982b) Non-histone proteins and long-range organization of Hela interphase DNA. J Mol Biol 156:325–344
Lewis CD, Laemmli UK (1982) Higher order metaphase chromosome structure: evidence for metalloprotein interactions. Cell 29:171–181
Lohka MJ, Masui Y (1984) Roles of cytosol and cytoplasmic particles in nuclear envelope assembly and sperm pronuclear formation in cell-free preparations from amphibian eggs. J Cell Biol 98:1222–1230
Loir M, Bouvier D, Fornells M, Lanneau M, Subirana JA (1985) Interactions of nuclear proteins with DNA, during sperm differentiation in the ram. Chromosoma 92:304–312
MacGregor HC, Walker MH (1973) The arrangement of chromosomes in nuclei of sperm from Plethodontid salamanders. Chromosoma 40:243–262
Marsden MPF, Laemmli UK (1979) Metaphase chromosome structure: Evidence for a radial loop model. Cell 17:849–858
Marushige K, Marushige Y, Wong TK (1976) Complete displacement of somatic histones during transformation of spermatid chromatin: A model experiment. Biochemistry 15:2047–2053
McGhee J (1986) The structure of interphase chromatin. In: Risley M (ed) Chromosome structure and function. Van Nostrand-Reinhold, New York, pp 2–38
McMaster-Kaye R, Kaye JS (1980) Organization of chromatin during spermiogenesis: Beaded fibers, partly beaded fibers, and loss of nucleosomal structure. Chromosoma 77:41–56
Miller OJ, Bakken AH (1972) Morphological studies of transcription. Acta Endocrinol Suppl 168:155–177
Mirkovitch J, Mirault M-E, Laemmli UK (1984) Organization of the higher-order chromatin loop: Specific DNA attachment sites on nuclear scaffold. Cell 39:223–232
Moses MJ, Solari AJ (1976) Positive contrast staining and protected drying of surface spreads: electron microscopy of the synaptonemal complex by a new method. J Ultrastruct Res 54:109–114
Muñoz-Guerra S, Azorin M, Casas MT, Marcet X, Maristany MA, Roca J, Subirana JA (1982) Structural organization of sperm chromatin from the fish Carassius auratus. Exp Cell Res 137:47–53
Oakley BR, Kirsch DR, Morris NR (1980) A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem 105:361–363
Palau J, Ruiz-Carrillo A, Subirana JA (1969) Histones from sperm of the sea urchin Arbacia lixula. Eur J Biochem 7:209–213
Paulson JR, Laemmli UK (1977) The structure of histone-depleted metaphase chromosomes. Cell 12:817–828
Phelan JJ, Subirana JA, Cole RD (1972) An unusual group of lysine-rich histones from gonads of a sea cucumber, Holothuria tubulosa. Eur J Biochem 31:63–68
Pruslin FH, Rodman TC (1983) Proteins of de-membraned protamine-depleted mouse sperm. Exp Cell Res 144:115–126
Risley MS (1977) Basic chromatin protein changes during spermatogenesis in Xenopus laevis. PhD Dissertation, The City University of New York, New York
Risley MS (1983) Spermatogenic cell differentiation in vitro. Gamete Res 4:331–346
Risley MS, Eckhardt RA (1979) Dissociation and separation of Xenopus laevis spermatogenic cells. J Exp Zool 207:93–106
Risley MS, Eckhardt RA (1981) H1 histone variants in Xenopus laevis. Dev Biol 84:79–87
Simon RH, Camerino-Otero RD, Felsenfeld G (1978) An octamer of histones H3 and H4 forms a compact complex with DNA of nucleosome size. Nucleic Acids Res 5:4805–4818
Sobhon P, Tanphaichitr N, Chutatape C, Vongpayabal P, Panuwatsuk W (1982) Electron microscopic and biochemical analyses of the organization of human sperm chromatin decondensed with sarkosyl and dithiothreitol. J Exp Zool 223:277–290
Spadafora C, Bellard M, Compton JL, Chambon P (1976) The DNA repeat length in chromatins from sea urchin sperm and gastrule cells are markedly different. FEBS Lett 69:281–285
Stick R, Schwarz H (1982) The disappearance of the nuclear lamina during spermatogenesis: an electron microscopic and immunofluorescence study. Cell Diff 11:235–243
Subirana JA (1983) Nuclear proteins in spermatozoa and their interactions with DNA. In: Andre J (ed) The sperm cell. Martinus Nijhoff, The Hague, pp 197–213
Vogelstein B, Pardoll DM, Coffey DS (1980) Supercoiled loops and eukaryotic DNA replication. Cell 22:79–85
Wagner TE, Yun JS (1981) Human sperm chromatin has a nucleosomal structure. Arch Androl 7:251–257
Walker MH (1971) Studies on the arrangement of nucleoprotein in elongate sperm heads. Chromosoma 34:340–354
Wallace RA, Jared DW, Dumont JN, Sega MW (1973) Protein incorporation by isolated amphibian oocytes. III. Optimum incubation conditions. J Exp Zool 184:321–334
Young RJ, Sweeney K (1979) The strutural organization of sperm chromatin. Gamete Res 2:265–282
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Risley, M.S., Einheber, S. & Bumcrot, D.A. Changes in DNA topology during spermatogenesis. Chromosoma 94, 217–227 (1986). https://doi.org/10.1007/BF00288496
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00288496