Abstract
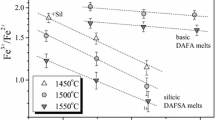
The redox ratio of iron is used as an indicator of solution properties of silicate liquids in the system (SiO−Al2O3−K2O−FeO−Fe2O3−P2O5). Glasses containing 80–85 mol% SiO2 with 1 mol% Fe2O3 and compositions covering a range of K2O/Al2O3 were synthesized at 1400°C in air (fixed fO2). Variations in the ratio FeO/FeO1.5 resulting from the addition of P2O5 are used to determine the solution behavior of phosphorus and its interactions with other cations in the silicate melt. In 80 mol% SiO2 peralkaline melts the redox ratio, expressed as FeO/FeO1.5, is unchanged relative to the reference curve with the addition of 3 mol% P2O5. Yet, the iron redox ratio in the 85 mol% SiO2 potassium aluminosilicate melts is decreased relative to phosphorus-free liquids even for small amounts of P2O5 (0.5 mol%). The redox ratio in peraluminous melts is decreased relative to phosphorus- free liquids at P2O5 concentrations of 3 mol%. In peraluminous liquids, complexing of both Fe+3−O−P+5 and Al+3−O−P+5 occur. The activity coefficient of Fe+3 is decreased because more ferric iron can be accommodated than in phosphorus-free liquids. In peralkaline melts, there is no evidence that P+5 is removing K+ from either Al+3 or Fe+3 species. In chargebalanced melts with 3 mol% Fe2O3 and very high P2O5 concentrations, phosphorus removes K+ from K−O−Fe+3 complexes resulting in a redox increase. P2O5 should be accommodated easily in peraluminous rhyolitic liquids and phosphate saturation may be suppressed relative to metaluminous rhyolites. In peralkaline melts, phosphate solubility may increase as a result of phosphorus complexing with alkalis. The complexing stoichiometry may be variable, however, and the relative influence of peralkalinity versus temperature on phosphate solubility in rhyolitic melts deserves greater attention.
Similar content being viewed by others
References
Barberi F, Ferrara G, Santacroce R, Treuil M, Varet J (1975) A transitional basalt-pantellerite sequence of fractional crystallization, the Boina centre (Afar rift, Ethiopia). J Petrol 16:22–56
Bizouard H, Barberi F, Varet J (1980) Mineralogy and petrology of Erta Ale and Boina volcanic series, Afar rift, Ethiopia. J Petrol 21:401–436
Bowen NL (1945) Phase equilibria bearing on the origin and differentiation of the alkaline rocks. Am J Sci 243A:75–89
Brown GE Jr, Calas G, Waychunas GA, Petiau J (1988) X-ray absorption spectroscopy and its applications in mineralogy and geochemistry. In: Hawthorne FC (ed) Spectroscopic methods in mineralogy and geology. Reviews in Mineralogy 18:431–512
Chase GD, Rabinowitz JL (1967) Principles of radioisotope methodology (3rd edn.) Burgess Publ Co, Minneapolis MN, pp 75–108
Civetta L, Cornette Y, Crisci G, Gillot PY, Orsi G, Requejo CS (1984) Geology, geochronology and chemical evolution of the island of Pantelleria. Geol Mag 121:541–668
Dickenson MP (1984) The structural role and homogeneous redox equilibria of iron in peraluminous, metaluminous, and peralkaline silicate melts. PhD Thesis Brown University, Providence, USA
Dickenson MP, Hess PC (1986a) The structural role and homogeneous redox equilibria of iron in peraluminous, metaluminous and peralkaline silicate melts. Contrib Mineral Petrol 92:207–217
Dickenson MP, Hess PC (1986b) The structural role of Fe3+, Ga3+, Al3+ and homogenous iron redox equilibria in K2O−Al2O3−Ga2O3−SiO2−Fe2O3−FeO melts. J Non-Cryst Solids 86:303–310
Dickenson MP, Hess PC (1981) Redox equilibria and the structural role of iron in alumino-silicate melts. Contrib Mineral Petrol 78:352–357
Dickinson JE, Hess PC (1985) Rutile solubility and titanium coordination in silicate melts. Geochim Cosmochim Acta 49:2289–2296
Dupree R, Holland D, Mortuza MG, Collins JA, Lockyer MWG (1989) Magic-angle spinning NMR of alkali phospho-aluminosilicate glasses. J Non-Crystal Solids 112:111–119
Dupree R, Holland D, Mortuza MG (1988a) The role of small amounts of P2O5 in the structure of alkali disilicate glasses. Phys Chem Glasses 29:18–21
Dupree R, Holland D, Mortuza MG, Collins JA, Lockyer MWG (1988b) An MAS NMR study of network-cation coordination in phosphosilicate glasses. Non-Crystal Solids 106:403–407
Dyar MD (1985) A review of Mössbauer data on inorganic glasses: the effects of composition on iron valency and coordination. Am Mineral 70:304–316
Ellison AJG, Hess PC (1988) Peraluminous and peralkaline effects upon “monazite” solubility in high-silica liquids. EOS 69:498
Fox KE, Furukawa T, White WB (1982) Transition metal ions in silicate melts. Part 2. Iron in sodium silicate glasses. Phys Chem Glasses 23:169–178
Gan H, Hess PC (1992) Phosphorus speciation in potassium aluminosilicate melts. Am Mineral 177:501–512
Gan H, Hess PC, Kirkpatrick RJ (1992) Heirarchy of interactions between Si−B−K−P in granitic melts. EOS 73:357
Green TH, Watson EB (1982) Crystallization of apatite in natural magmas under high-pressure, hydrous conditions, with particular reference to “orogenic” rock series. Contrib Mineral Petrol 79:96–105
Gwinn RE (1991) The peralkaline effect in rhyolitic melts: iron titanium oxide solubility; Phosphorus solution properties and the iron redox equilibrium; phase equilibria in a peralkaline rhyolite. PhD Thesis, Brown University, Providence, USA
Gwinn R, Hess PC (1989) Iron and titanium solution properties in peraluminous and peralkaline rhyolitic liquids. Contrib Mineral Petrol 101:326–338
Harrison TM, Watson (1984) The behavior of apatite during crustal anatexis: equilibrium and kinetic considerations. Geochim Cosmochim Acta 18:1467–1477
Hess PC (1991) The role of high field strength cations in silicate melts. In: Advances in physical chemistry, vol. 9, Springer, New York Berlin Heidelberg, pp 152–191
Hess PC, Horzempa P, Rutherford MJ, Devine J (1990) Phosphate equilibria in lunar basalts. (Abstr) Lunar Planet Sci Conf XXI:505–506
Hildreth W (1979) The Bishop Tuff: evidence for the origin of compositional zonation in silicic magma chambers. Geol Soc Am Spec Pap 180:43–75
Holz F, Johannes W, Pichavant M (1992) Effect of excess aluminum on phase relations in the system Qz-Ab-Or: experimental investigation at 2 kbar and reduced H2O activity. Eur J Mineral 4:137–152
Johnson MC (1990) Intensive parameters of volcanic magma systems from experimental amphibole/melt equilibria. PhD Thesis, Brown University, Providence, USA
Johnson CM, Lipman PW (1988) Origin of metaluminous and alkaline rocks of the Latir volcanic field, northern Rio Grande rift, New Mexico. Contrib Mineral Petrol 100:107–128
Kilinc A, Carmichael ISE, Rivers ML, Sack RO (1983) The ferricferrous ratio of natural silicate liquids equilibrated in air. Contrib Mineral Petrol 83:136–140
Kushiro I (1975) On the nature of silicate melts and its significance in magma gensis: regularities in the shift of liquidus boundaries involving olivine, pyroxene, and silica minerals. Am J Sci 275:411–431
Lacy ED (1963) Aluminum in glasses and in melts. Phys Chem Glasses 4:234–238
Levin EM, Robbins CR, McMurdie HF (1969) Phase diagrams for ceramists. 1969 Supplement. Am Ceram Soc Inc, Columbus Ohio
London D (1987) Internal differentiation of rare-element pegmatites: effects of boron, phosphorus, and fluorine. Geochim Cosmochim Acta 51:403–420
London D (1992) Phosphorus in S-type magmas: the P205 content of feldspars from peraluminous granites, pegmatites, and rhyolites. Am Mineral 77:126–145
Lu Y, Ding A, Tang Y (1988) Structural change of soda lime glass with minor P2O5 addition and heat treatment. J Non-Cryst Sol 106:391–394
Macdonald R, Davies GR, Bliss CM, Leat PT, Bailey DK, Smith RL (1987) Geochemistry of high-silica peralkaline rhyolites, Naivasha, Kenya Rift Valley. J Petrol 28:979–1008
Montel JM (1986) Experimental determination of the solubility of Ce-monazite in SiO2−Al2O3−K2O−Na2O melts at 800°C, 2 kbar, under H2O-saturated conditions. Geology 14:659–662
Mysen BO (1990) Interaction between phosphorous and iron oxides in silicate melts. Annual Report of the Director of the Geophysical Laboratory, Carnegie Instn Washington, 1989–1990, Geophysical Laboratory, Washington DC 66–75
Mysen BO, Virgo D (1989) Redox equilibria, structure, and properties of Fe-bearing aluminosilicate melts: relationships among temperature, composition, and oxygen fugacity in the system Na2O−Al2O3−SiO2−Fe−O. Am Mineral 74:58–76
Mysen BO, Ryerson FJ, Virgo D (1981) The structural role of phosphorus in silicate melts. Am Mineral 66:106–117
Nelson C, Tallant DR (1984) Raman studies of sodium silicate glasses with low phosphate contents. Phys Chem Glasses 25:31–38
Nicholis J, Carmichael ISE (1969) Peralkaline acid liquids: a petrological study. Contrib Mineral Petrol 20:268–294
Paul A, Douglas RW (1965) Ferrous-ferric equilibrium in alkali silicate glasses. Phys Chem Glasses 6:207–211
Pichavant M, Montel JM (1988) Petrogenesis of a two-mica ignimbrite suite: the Macusani Volcanics, SE Peru. Trans Roy Soc Edinburgh: Earth Sci 73:197–207
Pichavant M, Verrara JV, Boulmet S, Brique L, Joron J-L, Juteau M, Marin L, Michard A, Sheppared SMF, Treuil M, Vernet M (1987) The Macusani glasses, SE Peru: evidence of chemical fractionation in peraluminous magmas. In: Mysen BO (ed) Magmatic processes: physicochemical principles. Geochem Soc Spec Publ 1:359–373
Risbud SH, Kirkpatrick RJ, Taglialavore AP, Montez B (1987) Solid-state NMR evidence of 4-, 5-, and 6-fold aluminum sites in roller-quenched SiO2−Al2O3 glasses. J Am Ceram Soc 70:C10-C12
Ryerson FJ (1985) Oxide solution mechanisms in silicate melts: systematic variations in the activity coefficient of SiO2. Geochim Cosmochim Acta 49:637–649
Ryerson FJ, Hess PC (1980) The role of P2O5 in silicate melts. Geochim Cosmochim Acta 44:611–624
Ryerson FJ, Hess PC (1978) Implications of liquid-liquid distribution coefficients to mineral-liquid partitioning. Geochim Cosmochim Acta 42:921–932
Sack RO, Carmichael ISE, Rivers M, Ghiorso MS (1981) Ferricferrous equilibria in natural silicate liquids at 1 bar. Contrib Mineral Petrol 75:369–376
Skoog DA, West DM (1982) Fundamentals of analytical chemistry. CBS College Publishing, New York, pp 39–90
Tallant DR, Nelson C (1986) Raman investigation of glass structures in the Na2O−SiO2−P2O5−Al2O3 system. Phys Chem Glasses 27:75–79
Thornber CR, Roeder PL, Foster JR (1980) The effect of composition on the ferric-ferrous ratio in basaltic liquids at atmospheric pressure. Geochim Cosmochim Acta 44:525–532
Visser W, Koster van Gross AF (1979) Effects of P2O5 and TiO2 on liquid immiscibility in the system K2O−FeO−Al2O3−SiO2. Am J Sci 279:970–989
Watson EB (1976) Two-liquid partition coefficients: experimental data and geochemical implications. Contrib Mineral Petrol 56:119–134
Watson EB (1979a) Apatite saturation in basic to intermediate magmas. Geophys Res Lett 6:937–940
Watson EB (1979b) Zircon saturation in felsic liquids: experimental data and applications to trace element geochemistry. Contrib Mineral Petrol 70:407–419
Watson EB, Capobianco CJ (1981) Phosphorus and the rare earth elements in felsic magmas: an assessment of the role of apatite. Geochim Cosmochim Acta 45:2349–2358
Waychunas GA, Brown GE, Ponader CW, Jackson WE (1988) Evidence from X-ray absorption for network-forming Fe+2 in molten alkali silicates. Nature 332:251–253
Wells AF (1984) Structural inorganic chemistry. Clarendon Press, Oxford, UK
White AJR, Chappell BW (1988) Some supracrustal (S-type) granites of the Lachlan Fold Belt. Trans R Soc Edinburgh: Earth Sci 73:169–181
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gwinn, R., Hess, P.C. The role of phosphorus in rhyolitic liquids as determined from the homogeneous iron redox equilibrium. Contr. Mineral. and Petrol. 113, 424–435 (1993). https://doi.org/10.1007/BF00286932
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00286932