Abstract
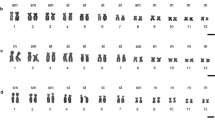
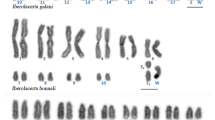
The karyotype of the Korean frog Rana dybowskii with its pattern of C-band heterochromatin distribution was numerically analyzed. There are 2n=24 chromosomes in the karyotype representing a reduction in number from the typical 2n=26 chromosome karyotype of Rana. The karyotype shows other evidence of reorganization relative to 26-chromosome species. The chromosomes grade smoothly in size from largest to smallest without the two size classes that are characteristic for 26-chromosome species. In contrast to many 26-chromosome species, there are few centromeric C-bands but many interstitial ones. C-bands for each homologous chromosome pair are distinctive. A prominent secondary constriction is located on one of the smallest chromosomes, chromosome 11, in a position similar to that seen in most 26-chromosome species. The karyotype of R. dybowskii is compared to those of other species of Rana known to have 2n=24 chromosomes; it is most similar to that of R. chensinensis, less so that of R. ornativentris and less still to that of R. arvalis in terms of the positions of centromeres and secondary constricitions. C-bands as well as secondary constrictions in the karyotypes of these frogs show evidence of chromosomal homosequentiality. The process and possible consequences of chromosome number reduction from an ancestral 26-chromosome karyotype is also evident in the karyotypes of these closely allied palearctic frogs. Pericentric inversions followed by fusion of two small elements apparently produced a new chromosome, chromosome 6, occurring originally among northeast Asian populations.
Similar content being viewed by others
References
Bachmann K, Nishioka M (1978) Genome size and nuclear size in palearctic frogs (Rana). Copeia 1978:225–229
Bogart JP (1973) Method for obtaining chromosomes. Caldasia 11:29–40
Bogart JP (1981) Chromosome studies in Sminthillus from Cuba and Eleutherodactylus from Cuba and Puerto Rico (Anura, Leptodactylidae). Life Sci Contrib, Royal Ontario Museum 129:1–22
Bogart JP, Tandy M (1981) Chromosome lineages in African ranoid frogs. Monit Zool Ital NS Suppl 15:55–91
Borkin LJ, Belimov GT, Sedalischev VT (1981) On the distribution of frogs genus Rana in Yakutia. In: Borkin LJ, (ed.) Herpetological investigations in Siberia and the Far East. Leningrad, Acad Sci USSR, Zool Inst pp 18–24
Green DM, Bogart JP, Anthony EH, Genner DL (1980) An interactive, micro-computer based karyotype analysis system for phylogenetic cytotaxonomy. Comput Biol Med 10:219–227
Green DM, Wasserman AO, Bogart JP (1981) Karyotypes of the frogs Rana septentrionalis and R. virgatipes. Copeia 1981:879–882
Haertel JD, Owczarzak A, Storm RM (1974) A comparative study of the chromosomes from five species of the genus Rana (Amphibia, Salientia). Copeia 1974:109–114
Kawamura T (1962) On the names of some Japanese frogs. J Sci Hiroshima Univ, Ser B 20:181–193
Kwamura T, Nishioka M (1973) Superiority of anuran amphibians as experimental materials. Exp Anim 22: (suppl); 115–126
Kawamura T, Nishioka M (1977) Aspects of the reproductive biology of Japanese anurans. In: Taylor DH, Guttman SI (eds.) The reproductive biology of amphibians. Plenum Publishing Corp New York pp 103–130
Kawamura T, Nishioka M, Ueda H (1981) Interspecific hybrids among Japanese, Formosan, European and American brown frogs. Sci Rep, Laboratory Amphib Biol, Hiroshima Univ 5:195–323
Kezer J, Sessions SK (1979) Chromosome variation in the plethodontid salamander Aneides ferreus. Chromosoma 71:65–80
King M (1980) C-banding studies on Australian hylid frogs: Secondary constriction structure and the concept of euchromatin transformation. Chromosoma 80:191–217
Kobayashi M (1962) Studies on reproductive isolation mechanisms in brown frogs II. Hybrid sterility. J Sci, Hiroshima Univ Ser B 20:157–179
Kuramoto M (1972) Karyotypes of the six species of frogs (genus Rana) endemic to the Ryukyu Islands. Caryologia 25:547–559
Kuramoto M (1974) Experimental hybridization between the brown frogs of Taiwan, the Ryukyu Islands and Japan. Copeia 1974:815–822
Kuramoto M (1979a) Interspecific hybridization between brown frogs, Rana okinavana female and Rana chensinensis male. Bull Fukuoka Univ Educ 28:45–48
Kuramoto M (1979b) Karyotypes of several frogs from Korea, Taiwan and the Philippines. Experientia 39:826–827
Macgregor H, Sherwood S (1979) The nucleolus organizers of Plethodon and Aneides located by in situ nucleic acid hybridization with Xenopus 3H-ribosomal RNA. Chromosoma 72:271–280
Mancino G, Ragghianti M, Bucci-Innocenti S (1977) Cytotaxonomy and cytogenetics in European newt species. In: Taylor DH, Guttman SI (eds.) The reproductive biology of amphibians. Plenum Publishing Corp New York pp 411–447
Morescalchi A (1968) Initial cytotaxonomic data on certain families of amphibious anura (Diplasiocoela) after Noble. Experientia 24:280–283
Morescalchi A (1973) Amphibia. In: Chiarelli AB, Capanna E eds. Cytotaxonomy and vertebrate evolution. Academic Press, New York pp 233–347
Morescalchi A (1979) New developments in vertebrate cytotaxonomy I. Cytotaxonomy of the amphibians. Genetics 50:179–193
Morescalchi A (1981) Karyology of the main groups of African frogs. Monit Zool Ital NS. 15: (Suppl), 41–53
Schmid M (1978a) Chromosome banding in amphibia I: Constitutive heterochromatin and nucleolus organizer regions in Bufo and Hyla. Chromosoma 66:361–388
Schmid M (1978b) Chromosome banding in Amphibia II: Constitutive heterochromatin and nucleolus organizer regions in Ranidae, Microhylidae and Rhacophoridae. Chromosoma 68:131–148
Seto T (1965) Cytogenetic studies in lower vertebrates II. Karyological studies of several species of frogs (Ranidae). Cytologia 30:437–446
Shannon FA (1956) The reptiles and amphibians of Korea. Herpetologica 12:22–49
Vitteli L, Batistoni R, Andonico F, Nardi I, Barsacchi-Pilone G (1982) Chromosomal localization of 18S + 28S and 5S ribosomal RNA genes in evolutionarily diverse anuran amphibians. Chromosoma 84:475–491
Ullerich FH (1967) Weitere Untersuchungen über Chromosomenverhältnisse und DNS-Gehalt bei Anuren (Amphibia). Chromosoma 21:345–368
Ward OG (1977) Dimorphic nucleolar organizer regions in the frog Rana blairi. Can J Genet Cytol 19:51–57
Wu Zhengan (1981) Karyotype of Rana chensinensis from Beijing. Acta Genet Sinica 8:138–144
Wu Zhengan (1982) Somatic chromosome of Hashima-frog. Acta Zool Sinica 28:23–27
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Green, D.M. Evidence for chromosome number reduction and chromosomal homosequentiality in the 24-chromosome Korean frog Rana dybowskii and related species. Chromosoma 88, 222–226 (1983). https://doi.org/10.1007/BF00285624
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00285624