Abstract
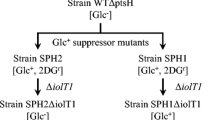
The glucose kinase gene (glkA-ORF3) of Streptomyces coelicolor A3(2) plays an essential role in glucose utilisation and in glucose repression of a variety of genes involved in the utilisation of alternative carbon sources. These genes include dagA, which encodes an extracellular agarase that permits agar utilisation. Suppressor mutants of glkA-ORF3 deletion strains capable of utilising glucose (Glc+) arise at a frequency of about 10−5 on prolonged incubation. The Glc+ phenotype of the mutants is reversible (at a frequency of about 10−3) and reflects either the activation of a normally silent glucose kinase gene or the modification of an existing sugar kinase. Although the level of glucose kinase activity in the Glc+ supressor mutants is similar to that in the glkA + parental strain, glucose repression of dagA remains defective. Expression of the glucose kinase gene of Zymomonas mobilis in glkA-ORF3 mutants restored glucose utilisation, but not glucose repression of dagA. Over-expression of glkA-ORF3 on a high-copy-number plasmid failed to restore glucose repression of dagA in glkA-ORF3 mutants and led to loss of glucose repression of dagA in a glkA + strain. These results suggest that glucose phosphorylation itself is not sufficient for glucose repression and that glkA-ORF3 plays a specific regulatory role in triggering glucose repression in S. coelicolor A3(2).
Similar content being viewed by others
References
Angell S (1992) The glucose kinase gene of Streptomyces coelicolor A3(2) and its role in glucose repression. PhD Thesis, University of East Anglia, Norwich
Angell S, Schwarz E, Bibb MJ (1992) The glucose kinase gene of Streptomyces coelicolor A3(2): its nucleotide sequence, transcriptional analysis and role in glucose repression. Mol Microbiol 6:2833–2844
Barnell WO, Yi KC, Conway T (1990) Sequence and genetic organization of a Zymomonas mobilis gene cluster that encodes several enzymes of glucose metabolism. J Bacteriol 172:7227–7240
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Buttner MJ, Lewis CG (1992) Construction and characterization of Streptomyces coelicolor A3(2) mutants that are multiply deficient in the nonessential hrd-encoded RNA polymerase sigma factors. J Bacteriol 174:5165–5167
Buttner MJ, Fearnley IM, Bibb MJ (1987) The agarase gene (dagA) of Streptomyces coelicolor A3(2): nucleotide sequence and transcriptional analysis. Mol Gen Genet 209:101–109
Chatterjee S, Vining LC (1982) Catabolite repression in Streptomyces venezuelae. Induction of β-galactosidase, chloramphenicol production, and intracellular cyclic adenosine 3′,5′-monophosphate concentrations. Can J Microbiol 28:311–317
Curtis SJ, Epstein W (1975) Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol 122:1189–1199
Dodd IB, Egan JB (1990) Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res 18:5019–5026
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Feinberg AP, Vogelstein B (1984) Addendum: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 137:266–267
Fernández R, Herrero P, Fernández E, Fernández MT, Lopez-Boado YS, Moreno F (1988) Autophosphorylation of yeast hexokinase PII. J Gen Microbiol 134:2493–2498
Fisher SH, Bruton CJ, Chater KF (1987) The glucose kinase gene of Streptomyces coelicolor and its use in selecting spontaneous deletions for desired regions of the genome. Mol Gen Genet 206:35–44
Fishman SE, Hershberger CL (1983) Amplified DNA in Streptomyces fradiae. J Bacteriol 155:459–466
Flores ME, Ponce E, Rubio M, Huitrón C (1993) Glucose and glycerol repression of α-amylase in Streptomyces kanamyceticus and isolation of deregulated mutants. Biotechnol Lett 15:595–600
Hall BG (1990) Directed evolution of a bacterial operon. BioEssays 12:551–558
Henderson PJF, Giddens RA, Jones-Mortimer MC (1977) Transport of galactose, glucose and their molecular analogues by Escherichia coli K12. Biochem J 162:309–320
Herrero P, Fernández R, Moreno F (1989) The hexokinase PII of Saccharomyces cerevisiae is a protein kinase. J Gen Microbiol 135:1209–1216
Hodgson DA (1980) Carbohydrate utilisation in Streptomyces coelicolor A3(2). PhD Thesis, University of East Anglia, Norwich
Hodgson DA (1982) Glucose repression of carbon source uptake in Streptomyces coelicolor A3(2) and its perturbation in mutants resistant to 2-deoxyglucose. J Gen Microbiol 128:2417–2430
Hodgson DA, Chater KF (1981) A chromosomal locus controlling extracellular agarase production by Streptomyces coelicolor A3(2) and its inactivation by chromosomal integration of plasmid SCP1. J Gen Microbiol 124:339–348
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H (1985) Genetic manipulation of Streptomyces: a laboratory manual. John Innes Foundation, Norwich
Hotter R, Eckhardt T (1988) Genetic manipulation. In: Goodfellow M, Williams ST, Mordarski M (eds) Actinomycetes in biotechnology. Academic Press, London, pp 89–184
Ikeda H, Seno ET, Bruton CJ, Chater KF (1984) Genetic mapping, cloning and physiological aspects of the glucose kinase gene of Streptomyces coelicolor. Mol Gen Genet 196:501–507
Janssen GR, Bibb MJ (1993) Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene 124:133–134
Katz E, Thompson CJ, Hopwood DA (1983) Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol 129:2703–2714
Lee AT, Cerami A (1987) Elevated glucose 6-phosphate levels are associated with plasmid mutations in vivo. Proc Natl Acad Sci USA 84:8311–8314
Lydiate DJ, Malpartida F, Hopwood DA (1985) The Streptomyces plasmid SCP2*: its functional analysis and development into useful cloning vectors. Gene 35:223–235
Ma H, Bloom LM, Walsh CT, Botstein D (1989) The residual enzyme phosphorylation activity of hexokinase II mutants is correlated with glucose repression in Saccharomyces cerevisiae. Mol Cell Biol 9:5643–5649
MacNeil DJ, Gewain KM, Ruby CL, Dezeny G, Gibbons PH, MacNeil T (1992) Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 111:61–68
Murray MG (1986) Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping. Anal Biochem 158:165–170
Novotná J, Hostálek Z (1985) Phosphorylation of hexoses in Streptomyces aureofaciens: evidence that the phosphoenolpyruvate:sugar phosphotransferase system is not operative. FEMS Microbiol Lett 28:347–350
Prior C, Mamessier P, Fukuhara H, Chen XJ, Wesolowski-Louvel M (1993) The hexokinase gene is required for transcriptional regulation of the glucose transporter gene RAG1 in Kluyveromyces lactis. Mol Cell Biol 13:3882–3889
Rose M, Albig W, Entian K-D (1991) Glucose repression in Saccharomyces cerevisiae is directly associated with hexose phosphorylation by hexokinases PI and PII. Eur J Biochem 199:511–518
Sabater B, Sebastian J, Asensio C (1972) Identification and properties of an inducible and highly specific fructokinase from Streptomyces violaceoruber. Biochim Biophys Acta 284:414–420
Saier MH (1989) Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate:sugar phosphotransferase system. Microbiol Rev 53:109–120
Saier MH (1991) A multiplicity of potential carbon catabolite repression mechanisms in prokaryotic and eukaryotic microorganisms. New Biologist 3:1137–1147
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Saunders JR (1989) Modulating bacterial virulence. Nature 338:622–623
Scopes RK, Testolin V, Stoter A, Griffiths-Smith K, Algar EM (1985) Simultaneous purification and characterization of glucokinase, fructokinase and glucose-6-phosphate dehydrogenase from Zymomonas mobilis. Biochem J 228:627–634
Seno ET, Chater KF (1983) Glycerol catabolic enzymes and their regulation in wild-type and mutant strains of Streptomyces coelicolor A3(2). J Gen Microbiol 129:1403–1413
Sierkstra LN, Sillje HHW, Verbakel JMA, Verrips CT (1993) The glucose-6-phosphate-isomerase reaction is essential for normal glucose repression in Saccharomyces cerevisiae. Eur J Biochem 214:121–127
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Stibitz S, Aaronson W, Monack D, Falkow S (1989) Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature 338:266–269
Strauch E, Takano E, Baylis HA, Bibb MJ (1991) The stringent response in Streptomyces coelicolor A3(2). Mol Microbiol 5:289–298
Virolle MJ, Bibb MJ (1988) Cloning, characterization and regulation of an α-amylase gene from Streptomyces limosus. Mol Microbiol 2:197–208
Virolle MJ, Long CM, Chang S, Bibb MJ (1988) Cloning, characterization and regulation of an α-amylase gene from Streptomyces venezuelae. Gene 74:321–334
Weiser JN, Love JM, Moxon ER (1989) The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell 59:657–665
Wong HC, Ting Y, Lin HC, Reichert F, Myambo K, Watt KWK, Toy PL, Drummond RJ (1991) Genetic organization and regulation of the xylose degradation genes in Streptomyces rubiginosus. J. Bacteriol 173:6849–6858
Yannisch-Peron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mpl8 and pUC19 vectors. Gene 33:103–119
Zieg J, Silverman M, Hilmen M, Simon M (1977) Recombinational switch for gene expression. Science 196:170–172
Author information
Authors and Affiliations
Additional information
Communicated by A. Kondorosi
Rights and permissions
About this article
Cite this article
Angell, S., Lewis, C.G., Buttner, M.J. et al. Glucose repression in Streptomyces coelicolor A3(2): a likely regulatory role for glucose kinase. Molec. Gen. Genet. 244, 135–143 (1994). https://doi.org/10.1007/BF00283514
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00283514