Summary
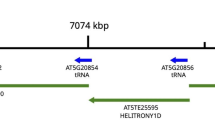
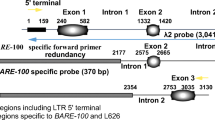
We have examined the organization of tRNATyr genes in three ecotypes of Arabidopsis thaliana, a plant with an extremely small genome of 7 × 107 bp. Three tRNATyr gene-containing EcoRI fragments of 1.5 kb and four fragments of 0.6, 1.7, 2.5 and 3.7 kb were cloned from A. thaliana cv. Columbia (Col-O) DNA and sequenced. All EcoRl fragments except those of 0.6 and 2.5 kb comprise an identical arrangement of two tRNATyr genes flanked by a tRNASer gene. The three tRNA genes have the same polarity and are separated by 250 and 370 bp, respectively. The tRNATyr genes encode the known cytoplasmic tRNAGψA Tyr. Both genes contain a 12 by long intervening sequence. Densitometric evaluation of the genomic blot reveals the presence of at least 20 copies, including a few multimers, of the 1.5 kb fragment in Col-O DNA, indicating a multiple amplification of this unit. Southern blots of EcoRl-digested DNA from the other two ecotypes, cv. Landsberg (La-O) and cv. Niederzenz (Nd-O) also show 1.5 kb units as the major hybridizing bands. Several lines of evidence support the idea of a strict tandem arrangement of this 1.5 kb unit: (i) Sequence analysis of the EcoRI inserts of 2.5 and 0.6 kb reveals the loss of an EcoRI site between 1.5 kb units and the introduction of a new EcoRI site in a 1.5 kb dimer. (ii) Complete digestion of Col-O DNA with restriction enzymes which cleave only once within the 1.5 kb unit also produces predominantly 1.5 kb fragments. (iii) Partial digestion with EcoRI shows that the 1.5 kb fragments indeed arise from the regular spacing of the restriction sites. The high degree of sequence homology among the 1.5 kb units, ranging from 92% to 99%, suggests that the tRNASer/tRNATyr cluster evolved 1–5 million years ago, after the Brassicaceae diverged from the other flowering plants about 5–10 million years ago.
Similar content being viewed by others
References
Arnold GJ, Schmutzler C, Thomann U, van Tol H, Gross HJ (1986) The human tRNAVal gene family; organization, nucleotide sequences and homologous transcription of three singlecopy genes. Gene 44:287–297
Barciszewski J, Barciszewska M, Suter B, Kubli E (1985) Plant tRNA suppressors: in vivo readthrough properties and nucleotide sequence of yellow lupin seeds tRNATyr. Plant Sci 40:193–196
Beier H, Barciszewska M, Krupp G, Mitnacht R, Gross HJ (1984 a) UAG readthrough during TMV RNA translation: isolation and sequence of two tRNATyr with suppressor activity from tobacco plants. EMBO J 3:351–356
Beier H, Barciszewska M, Sickinger H-D (1984b) The molecular basis for the differential translation of TMV RNA in tobacco protoplasts and wheat germ extracts. EMBO J 3:1091–1096
Bennett MD, Smith JB, Heslop-Harrison JS (1982) Nuclear DNA amounts in angiosperms. Proc R Soc Lond [B] 216:179–199
Choffat Y, Suter B, Behra R, Kubli E (1988) Pseudouridine modification in tRNATyr anticodon is dependent upon presence, but independent upon size and sequence of the intron in eukaryotic tRNATyr genes. Mol Cell Biol 8:3332–3337
Clarkson SG, Kurer V (1976) Isolation and some properties of DNA coding for tRNA1 Met from Xenopus laevis. Cell 8:183–195
DeLotto R, Schedl P (1984) A Drosophila melanogaster transfer RNA gene cluster at the cytogenic locus 9013C. J Mol Biol 179:587–605
Dingermann T, Brechner T, Marschalek R, Amon-Böhm E, Welker DL (1989) tRNAGlu (GAA) genes from the cellular slime mold Dictyostelium discoideum. DNA 8:193–204
Francis MA, Dudock BS (1989) Nucleotide sequence of spinach cytoplasmic serine (IGA) tRNA. Nucleic Acids Res 17:79–96
Gerlach WL, Dyer TA (1980) Sequence organization of the repeating units in the nucleus of wheat which contain 5 S rRNA genes. Nucleic Acids Res 8:4851–4865
Gouilloud E, Clarkson SG (1986) A dispersed tyrosine tRNA gene from Xenopus laevis with high transcriptional activity in vitro. J Biol Chem 261:486–494
Green GA, Weil J-H (1989) Evolution of a plant nuclear tRNAPro gene cluster. Plant Mol Biol 13:727–730
Hattori M, Sakaki Y (1986) Dideoxy sequencing method using denatured plasmid templates. Anal Biochem 152:232–238
Hemleben V, Grierson D (1978) Evidence that in higher plants the 25S and 18S rRNA genes are not interspersed with genes for 5 S rRNA. Chromosoma 65:353–358
Hemleben V, Werts D (1988) Sequence organization and putative regulatory elements in the 5 S rRNA genes of higher plants (Vigna radiata and Matthiola incana). Gene 62:165–169
Johnson PF, Abelson J (1983) The yeast tRNATyr gene intron is essential for correct modification of its tRNA product. Nature 302:681–687
Kimura M (1977) Preponderance of synonymous changes as evidence for the neutral theory of molecular evolution. Nature 267:275–276
Leutwiler LS, Hough-Evans BR, Meyerowitz EM (1984) The DNA of Arabidopsis thaliana. Mol Gen Genet 194:15–23
Martin W, Gierl A, Saedler H (1989) Molecular evidence for preCretaceous angiosperm origins. Nature 339:46–48
Meyerowitz EM, Pruitt RE (1985) Arabidopsis thaliana and plant molecular genetics. Science 229:1214–1218
Miyata T, Yasunaga T, Nishida T (1980) Nucleotide sequence divergence and functional constraint in mRNA evolution. Proc Natl Acad Sci USA 77:7328–7332
Müller F, Clarkson SG (1980) Nucleotide sequence of genes coding for tRNAPhe and tRNATyr from a repeating unit of X. laevis DNA. Cell 19:345–353
Muller J (1981) Fossil pollen records of extinct angiosperms. Bot Rev 47:1–142
Olson MV, Montgomery DL, Hopper AK, Page GS, Horodyski F, Hall BD (1977) Molecular characterization of the tyrosine tRNA genes of yeast. Nature 267:639–641
Pruitt RE, Meyerowitz EM (1986) Characterization of the genome of Arabidopsis thaliana. J Mol Biol 187:169–183
Rafalski JA, Wiewiorowski M, Söll D (1982) Organization and nucleotide sequence of nuclear 5 S rRNA genes in yellow lupin (Lupinus luteus). Nucleic Acids Res 10:7635–7642
Robinson RR, Davidson N (1981) Analysis of a Drosphila tRNA gene cluster: two tRNALeu genes obtain intervening sequences. Cell 23:251–259
Rosen A, Sarid S, Daniel V (1984) Genes and pseudogenes in a reiterated rat tRNA gene cluster. Nucleic Acids Res 12:4893–4906
Roy KL, Cooke H, Buckland R (1982) Nucleotide sequence of a segment of human DNA containing the three tRNA genes. Nucleic Acids Res 10:7313–7322
Santos T, Zasloff M (1981) Comparative analysis of human chromosomal segments bearing nonallelic dispersed tRNA1 Met genes. Cell 23:699–709
Shibuya K, Noguchi S, Nishimura S, Sekiya T (1982) Characterization of a rat tRNA gene cluster containing the genes for tRNAAsp, tRNAGly and tRNAGlu, and pseudogenes. Nucleic Acids Res 10:4441–4448
Shortridge RD, Johnson GD, Craig LC, Pirtle IL, Pirtle RM (1989) A human tRNA gene heterocluster encoding threonine, proline and valine tRNAs. Gene 79:309–324
Southern E (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Stange N, Beier H (1986) A gene for the major cytoplasmic tRNATyr from Nicotiana rustica contains a 13 nucleotides long intron. Nucleic Acids Res 14:86–91
Stange N, Gross HJ, Beier H (1988) Wheat germ splicing endonuclease is highly specific for plant pre-tRNAs. EMBO J 7:3823–3828
Stutz F, Gouilloud E, Clarkson SG (1989) Oocyte and somatic tyrosine tRNA genes in Xenopus laevis. Genes Dev 3:1190–1198
Suter B, Kubli E (1988) tRNATyr genes of Drosophila melanogaster: expression of single-copy genes studied by S1 mapping. Mol Cell Biol 8:3322–3331
Underwood DC, Knickerbocker H, Gardner G, Condliffe DP, Sprague KU (1988) Silk gland-specific tRNAAla genes are tightly clustered in the silkworm genome. Mol Cell Biol 8:5504–5512
Valenzuela P, Venegas A, Weinberg F, Bishop R, Rutter WJ (1978) Structure of yeast phenylalanine-tRNA genes: an intervening DNA segment within the region coding for the tRNA. Proc Natl Acad Sci USA 75:190–194
Van Tol H, Beier H (1988) All human tRNATyr genes contain introns as a prerequisite for pseudouridine biosynthesis in the anticodon. Nucleic Acids Res 16:1951–1966
Voytas DF, Ausubel FM (1988) A copia-like transposable element family in Arabidopsis thaliana. Nature 336:242–244
Wolfe KH, Li WH, Sharp PM (1987) Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci USA 84:9054–9058
Yen PH, Davidson N (1980) The gross anatomy of a tRNA gene cluster at region 42A of the D. melanogaster chromosome. Cell 22:137–148
Author information
Authors and Affiliations
Additional information
Communicated by H. Saedler
Rights and permissions
About this article
Cite this article
Beier, D., Stange, N., Gross, H.J. et al. Nuclear tRNATyr genes are highly amplified at a single chromosomal site in the genome of Arabidopsis thaliana . Molec. Gen. Genet. 225, 72–80 (1991). https://doi.org/10.1007/BF00282644
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00282644