Abstract
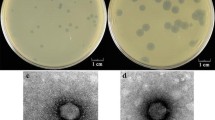
A recombinant lambda phage was identified in a Clostridium perfringens genomic library by means of its ability to hydrolyse the fluorescent substrate 4-methyl-umbelliferyl-β-d-glucosaminide, isolated and shown to encode an endo-β-N-acetylglucosaminidase. This enzyme, NagH, is also known as hyaluronidase, or Mu toxin, a putative virulence factor which is likely to act on connective tissue during gas gangrene. Nucleotide sequence analysis allowed the primary structure to be deduced and showed hyaluronidase to be a large exported protein of 114392 Daltons and an enzyme of this size, endowed with the corresponding activities, was partially purified from C. perfringens. Hyaluronidase seems to be organised into two domains, an N-terminal region comprising 700 amino acids bearing the active site and a 300-residue C-terminal segment, containing three copies of an extended motif. Two other reading frames, linked to nagH, also appear to encode proteins with sugar-binding motifs.
Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bairoch A (1992) A dictionary of protein sites and patterns. Nucleic Acids Res 20:2013–2018
Banas JA, Russel RRB, Ferretti J (1990) Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect Immun 58:667–673
Canard B, Cole ST (1989) Genome organisation of the anaerobic pathogen clostridium perfringens. Proc Natl Acad Sci USA 86:6676–6680
Canard B, Saint-Joanis B, Cole ST (1992) Genomic diversity and organisation of virulence genes in the pathogenic anaerobe Clostridium perfringens. Mol Microbiol 6:1421–1429
Chambers SP, Prior SE, Barstow DA, Minton NP (1988) The pMTL nic − cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene 68:139–149
Chaplin MF (1986) Monosaccharides. In: Chaplin MF, Kennedy JF (eds) Carbohydrate analysis. IRL Press, Oxford, pp 1–36
Chien SF, Yevich SJ, Li SC, Li YT (1975) Presence of endo-β-N-acetylglucosaminidase and protease activities in the commercial neuramidase preparations isolated from Clostridium perfringens. Biochem Biophys Res Commun 65:683–691
Cliver DO (1987) Foodborne disease in the United States 1946–1986. Int J Food Microbiol 4:269–277
Cole ST, Garnier T (1993) Molecular genetic studies of UV-inducible Bacteriocin production in Clostridium perfringens. In: Sebald M (ed) Genetics and Molecular Biology of Anaerobes. Springer-Verlag, New York, pp 248–254
Garnier T, Cole ST (1988) Complete nucleotide sequence and genetic organization of the bacteriocinogenic plasmid, pIP404, from Clostridium perfringens. Plasmid 19:134–150
Gilmore KS, Russel RRB, Ferretti J (1990) Analysis of the Streptococcus downei gtfs gene which specifies a glucosyltransferase that synthesizes soluble glucans. Infect Immun 58:2452–2458
Granum P (1980) Clostridium perfringens toxins involved in food poisoning. Int J Food Microbiol 10:101–112
Ito S, Muramatsu T, Kobata A (1975) Endo-β-N-Acetylglucosaminidases acting on carbohydrate moieties of glycoproteins: Purification and properties of two enzymes with different specificities from Clostridium perfringens. Arch Biochem Biophys 171:78–86
Leslie D, Faiweather N, Pickard D, Dougan G, Kehoe M (1989) Phospholipase C and haemolytic activities of Clostridium perfringens alpha-toxin cloned in Escherichia coli: sequence and homology with Bacillus cereus phospholipase C. Mol Microbiol 33:383–392
McDonel JL (1986) Toxins of Clostridium perfringens type A, B, C, D and E. In: Dorner F, Drews J (eds) Pharmacology of Bacterial Toxins. Pergamon Press, Oxford, pp 477–517
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, New York
Okabe A, Shimizu T, Hayashi H (1989) Cloning and sequencing of a phospholipase C gene of Clostridium perfringens. Biochem Biophys Res Commun 160:33–39
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85:2444–2448
Roggentin P, Rothe B, Lottspeich F, Schauer R (1988) Cloning and sequencing of a Clostridium perfringens sialidase gene. FEBS Lett 238:31–34
Rood JR, Cole ST (1991) Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol Rev 55:621–648
Saint-Joanis B, Garnier T, Cole ST (1989) Gene cloning shows the alpha toxin to Clostridium perfringens to contain both shingomyelinase and lecithinase activities. Mol Gen Genet 219:453–460
Shimizu T, Okaba A, Minami J, Hayashi H (1991) An upstream regulatory sequence stimulates expression of the perfringolysin O gene of Clostridium perfringens. Infect Immun 59:137–142
Sloan J, Warner TA, Scott PT, Bannam TL, Berryman DI, Rood JI (1992) Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid 27:207–219
Smith LDS (1979) Virulence factors of Clostridium perfringens. Rev Infect Dis 1:254–260
Staden R (1987) Computer handling of DNA sequencing projects. In: Bishop MJ, Rawlings CJ (eds) Nucleic acid and protein sequence analysis: A practical approach. IRL Press, Oxford, pp 173–217
Staden R (1988) Methods of define and locate motifs in sequences. Comp Appl Biosci 5:89–96
Tabor S, Richardson CC (1985) A bacteriophage T7 RNA polymerase: promoter for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA 82:1074–1078
Tai T, Yamashita K, Kobata A (1977) The substrate specificities of endo-β-N-acetylglucosaminidase CII, and H. Biochem Biophys Res Commun 78:434–441
Titball RW, Hunter SEC, Martin KL, Morris BC, Shuttleworth AD, Rubidge T, Anderson DW, Kelly DC (1989) Molecular cloning and nucleotide sequence of the alpha-toxin (phospholipase C) of Clostridium perfringens. Infect Immun 57:367–376
Titball RW, Yeoman H, Hunter SEC (1992) Gene cloning and organisation of the alpha-toxin of Clostridium perfringens. In: Sebald M (ed) Genetics and Molecular Biology of Anaerobes. Springer-Verlag, New York, pp 211–226
Tso JY, Siebel C (1989) Cloning and expression of the phospholipase C gene from Clostridium perfringens and Clostridium bifermentans. Infect Immun 57:468–476
Tweten RK (1988a) Cloning and expression in Escherichia coli of the perfringolysin 0 (theta-toxin) from Clostridium perfringens and characterization of the gene product. Infect Immun 56:3228–3234
Tweten RK (1988) Nucleotide sequence of the gene for perfringolysin O (theta-toxin) from Clostridium perfringens: significant homology with the genes for streptolysin O and pneumolysin. Infect Immun 56:3235–3240
Williamson R, Ward JB (1979) Characterization of the autolytic enzymes of Clostridium perfringens. J Gen Microbiol 114:349–354
Yamamoto K, Kadowaki S, Takegawa K, Kumagai H, Tochikura T (1986) Purification and characterization of endo-β-N-acetyl-glucosaminidase from a Flavobacterium sp. Agric Biol Chem 50:421–429
Author information
Authors and Affiliations
Additional information
Communicated by W. Goebel
Rights and permissions
About this article
Cite this article
Canard, B., Garnier, T., Saint-Joanis, B. et al. Molecular genetic analysis of the nagH gene encoding a hyaluronidase of Clostridium perfringens . Molec. Gen. Genet. 243, 215–224 (1994). https://doi.org/10.1007/BF00280319
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00280319