Abstract
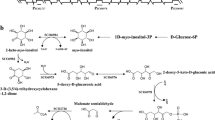
Mutations in four genes: sconA (formerly suA25meth, mapA25), sconB (formerly mapBl), sconC and sconD, the last two identified in this work, relieve a group of sulphur amino acid biosynthetic enzymes from methionine-mediated sulphur metabolite repression. Exogenous methionine has no effect on sulphate assimilation in the mutant strains, whereas in the wild type it causes almost complete elimination of sulphate incorporation. In both mutant and wild-type strains methionine is efficiently taken up and metabolized to S-adenosylmethionine, homocysteine and other compounds. scon mutants also show elevated levels of folate-metabolizing enzymes which results from the large pool of homocysteine found in these strains. The folate enzymes apear to be inducible by homocysteine and repressible by methionine (or Sadenosylmethionine).
Similar content being viewed by others
References
Apte BN, Bhavsar PN, Siddigi O (1974) The regulation of aryl sulphatase in Aspergillus nidulans. J Mol Biol 86:637–648
Arst HN Jr, Bailey CR (1977) The regulation of carbon metabolism in Aspergillus nidulans. In: Smith JE, Pateman JA (eds) Genetics and physiology of Aspergillus. Academic Press, London, pp 130–146
Arst HN Jr, Scazzocchio C (1985) Formal genetics and molecular biology of the control of gene expression in Aspergillus nidulans. In: Bennett JW, Lasure LL (eds) Gene manipulations in filamentous fungi. Academic Press, London, pp 309–343
Bradfield G, Somerfield P, Meyn T, Holby M, Babcock D, Bradley D, Segel IH (1970) Regulation of sulphate transport in filamentous fungi. Plant Physiol 46:720–727
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burton EG, Metzenberg RL (1972) Novel mutation causing derepression of several enzymes of sulphur metabolism in Neurospora crassa. J Bacteriol 109:140–151
Cai X-Y, Redfield B, Maxon M, Weissbach H, Brot N (1989) The effect of homocysteine on MetR regulation of metE, metR and metH expression in vitro. Biochem Biophys Res Commun 163:79–83
Cherest H, Eichler F, de Robichon-Szulmajster H (1969) Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J Bacteriol 97:328–336
Cherest H, Surdin-Kerjan Y, de Robichon-Szulmajster H (1971) Methionine-mediated repression in Saccharomyces cerevisiae: a pleiotropic regulatory system involving methionyl transfer ribonucleic acid and the product of gene eth2. J Bacteriol 106:758–772
Cherest H, Surdin-Kerjan Y, Antoniewski J, de Robichon-Szulmajster H (1973) S-adenosyl methionine mediated repression of methionine biosynthetic enzymes in Saccharomyces cerevisiae. J Bacteriol 114:928–933
Cherest H, Thao NN, Surdin-Kerjan Y (1985) Transcriptional regulation of the MET3 gene of Saccharomyces cerevisiae. Gene 34:269–281
Clutterbuck AJ (1984) Loci and linkage map of the filamentous fungus Aspergillus nidulans. Genet Maps 3:265–273
Cybis J, Natorff R, Lewandowska I, Prazmo W, Paszewski A (1988) Mutations affecting cysteine synthesis in Aspergillus nidulans: characterization and chromosome mapping. Genet Res 51:85–88
Dietrich PS (1972) MS Thesis. University of Wisconsin, Madison (cited after Paietta JV 1990)
Forbes E (1959) Use of mitotic segregation for assigning genes to linkage groups in Aspergillus nidulans. Heredity 13:67–80
Gajewski W, Litwifiska J (1968) Methionine loci and their suppressors in Aspergillus nidulans. Mol Gen Genet 102:210–220
Hanggi VJ, Littlefield JW (1974) Isolation and characterization of the multiple forms of the dihyrofolate reductase from methotrexate resistant hamster cells. J Biol Chem 249:1390–1397
Hanson MA, Marzluf GA (1973) Regulation of a sulphur-controlled protease in Neurospora crassa. J Bacteriol 116:785–789
Hastie AC (1970) Benlate-induced instability of Aspergillus diploids. Nature 226:771
Jacobson ES, Metzenberg RL (1977) Control of arylsulphatase in a serine auxotroph of Neurospora. J Bacteriol 130:1397–1398
Ketter JS, Marzluf GA (1988) Molecular cloning and analysis of the regulation of cys-14′, a structural gene of the sulphur regulatory circuit of Neurospora crassa. Mol Cell Biol 8:1504–1508
Kredich NM, Tomkins GM (1966) The enzymatic synthesis of l-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem 241:4955–4965
Łukaszkiewicz Z, Paszewski A (1976) Hyper-repressible operatortype mutant in sulphate permease gene of Aspergillus nidulans. Nature 259:337–338
Marzluf GA (1970) Genetic and metabolic controls for sulphate metabolism in Neurospora crassa: isolation and study of chromate-resistant and sulphate transport-negative mutants. J Bacteriol 102:716–721
Marzluf GA, Metzenberg RL (1968) Positive control by the cys-3 locus in regulation of sulphur metabolism in Neurospora. J Mol Biol 33:423–437
Metzenberg RL, Parson JW (1966) Altered repression of some enzymes of sulphur utilization in a temperature-conditional lethal mutant of Neurospora. Proc Natl Acad Sci USA 55:629–635
Morzycka E, Paszewski A (1979) Regulation of S-amino acids biosynthesis in Saccharomyces lipolytica. Mol Gen Genet 174:33–38
Nadolska-Lutyk J, Baliriska M, Paszewski A (1989) Interrelated regulation of sulphur-containing amino-acid biosynthetic en-zymes and folate-metabolizing enzymes in Aspergillus nidulans. Eur J Biochem 181:231–235
Paietta JV (1989) Molecular cloning and regulatory analysis of the arylsulphatase structural gene of Neurospora crassa. Mol Cell Biol 9:3630–3637
Paietta JV (1990) Molecular cloning and analysis of the scon-2 negative regulatory gene of Neurospora crassa. Mol Cell Biol 10:5207–5214
Paszewski A, Grabski J (1973) Studies on β-cystationase and Oacetylhomoserine sulphhydrylase as the enzymes of alternative methionine biosynthetic pathways in Aspergillus nidulans. Acta Biochim Polon 20:159–168
Paszewski A, Grabski J (1974) Regulation of S-amino acids biosynthesis in Aspergillus nidulans. Mol Gen Genet 132:307–320
Paszewski A, Grabski J (1975) Enzymatic lesions in methionine mutants of Aspergillus nidulans: role and regulation of an alternative pathway for cysteine and methionine synthesis. J Bacteriol 124:893–904
Paszewski A, Ono B-I (1992) Biosynthesis of sulphur amino acids in Saccharomyces cerevisiae: regulatory roles of methionine and S-adenosylmethionine reassessed. Curr Genet 22:273–276
Paszewski A, Praimo W, Landman-Balinska M (1977) Regulation of homocysteine metabolizing enzymes in Aspergillus nidulans. Mol Gen Genet 155:109–112
Paszewski A, Praimo W, Nadolska J, Regulski M (1984) Mutations affecting the sulphur assimilation pathway in Aspergillus nidulans: their effect on sulphur amino acid metabolism. J Gen Microbiol 130:1113–1121
Pienigzek NJ, Stgpien PP, Paszewski A (1973) An Aspergillus nidulans mutant lacking homocysteine (3-synthase: identity of l-serine sulphhydrylase with cystathionine β-synthase and its distinctness from O-acetyl-l-serine sulphhydrylase. Biochim Biophys Acta 297:37–47
Piotrowska M, Paszewski A (1990) A yeast with unusual sulphur amino acid meteabolism. J Gen Microbiol 136:2283–2286
Pontecorvo G, Roper JA, Hemmons LM, Macdonald KD, Bufton AW (1953) The genetics of Aspergillus nidulans. Adv Genet 5:141–238
Sangsoda S, Cherest H, Surdin-Kerjan Y (1985) The expression of MET25 gene of Saccharomyces cerevisiae is regulated transcriptionally. Mol Gen Genet 200:407–414
Scott JM, Spencer B (1968) Regulation of choline sulphatase synthesis and activity in Aspergillus nidulans. Biochem J 106:471–477
Scrimegour KG, Huennekens FM (1966) In: Bruns FH (ed) Handbuch der physiologisch- und patologisch-chemischen Analytik, vol 7b. Springer Verlag, Berlin, pp 181–208
Thomas D, Cherest H, Surdin-Kerjan Y (1989) Elements involved in S-adenosylmethionine-mediated regulation of the Saccharomyces cerevisiae MET25 gene. Mol Cell Biol 9:3292–3298
Urbanowski ML, Stauffer GV (1989) Role of homocysteine in metR-mediated activation of the metE and metH genes in Salmonella typhimurium and Escherichia coli. J Bacteriol 171:3277–3281
de Vito PC, Dreyfuss J (1964) Metabolic regulation of adenosine triphosphate sulphurylase in yeast. J Bacteriol 88:1341–1348
Author information
Authors and Affiliations
Additional information
Communicated by C.A.M.J.J. van den Hondel
Rights and permissions
About this article
Cite this article
Natorff, R., Balińska, M. & Paszewski, A. At least four regulatory genes control sulphur metabolite repression in Aspergillus nidulans . Molec. Gen. Genet. 238, 185–192 (1993). https://doi.org/10.1007/BF00279546
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00279546