Abstract
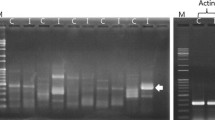
Gene fragments encoding serine proteases expressed in adult buffalo fly (Haematobia irritans exigua) were amplified from cDNA using generic oligonucleotide PCR primers, based on conserved residues surrounding the active-site His and Ser amino acids found in all serine proteases. The PCR product consisted of a broad band extending from about 450 by to 520 bp, which suggested that the PCR product actually consisted of numerous DNA fragments of slightly variable sizes. Seventeen independent clones of these fragments, each with an insert of approximately 480 bp, were digested with HaeIII. Comparison of restriction fragment patterns indicated that 13 of these clones harboured different PCR products. This was confirmed by DNA sequence analysis of 9 clones. Each of the sequenced clones contained an open reading frame which included structurally conserved regions characteristic of the serine protease superfamily. This study reveals the expression of a large and highly variable repertoire of serine proteases in adult buffalo fly. Importantly, these data also demonstrate the utility of such an approach in obtaining DNA probes for use in further investigations of gene family organization and expression, as well as providing recombinant antigens in the form of fusion proteins which may be used as candidates for vaccine production.
Similar content being viewed by others
References
Allingham PG, Kerlin RL, Tellam RL, Briscoe SJ, Standfast HA (1992) Passage of host immunoglobulin across the mid-gut epithelium into the haemolymph of blood-fed buffalo flies (Haematobia irritans exigua). J Insect Physiol 38:9–17
Barillas-Mury C, Graf R, Hagedorn HE, Wells MA (1991) cDNA and deduced amino acid sequences of a blood meal-induced trypsin from the mosquito Aedes aegypti. Insect Biochem 21:825–831
Bean G, Seifert A, Macqueen A, Doube BM (1987) Effect of insecticide treatment for control of buffalo fly on weight gains of steers in coastal central Queensland. Aust J Exp Agric 27:329–334
Ben-Yakir D (1989) Quantitative studies of host immunoglobulin in the haemolymph of ticks (Acari). J Med Entomol 26:243–246
Borovsky D (1986) Proteolytic enzymes and blood digestion in the mosquito, Culex nigripalpus. Arch Insect Biochem Physiol 3:147–160
Briegel H, Lea AO (1975) Relationship between protein and proteolytic activity in the midgut of mosquitoes. J Insect Physiol 21:1597–1604
Brown JR, Hartley BS (1966) Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J 101:214–228
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium-thyrocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Davis CA, Riddell DC, Higgins MJ, Holden JJA, White BN (1985) A gene family in Drosophila melanogaster coding for trypsin-like enzymes. Nucleic Acids Research 13:6605–6619
DeLotto R, Spierer P (1986) A gene required for the specification of dorsal-ventral pattern in Drosophila appears to encode a serine protease. Nature 323:688–692
D'Onofrio G and Bernardi G (1992) A universal compositional correlation among codon positions. Gene 110:81–88
Elizur A, Vacek AT, Howells AJ (1990) Cloning and characterisation of the white and topaz eye colour genes from the sheep blowfly Lucilia cuprina. J Mol Evol 30:347–358
Emi M, Nakamura Y, Ogawa M, Yamamoto T, Nishide T, Utari T, Matsubara K (1986) Cloning, characterisation and nucleotide, sequences of his cDNAs encoding human pancreatic trypsinogens. Gene 41:305–310
Ericson ML (1990) Quick DNA recovery from agarose gels by ultracentrifuge run. Trends Genet 6:278
Felix CR, Betschart B, Billingsley PF, Freyvogel TA (1991) Postfeeding induction of trypsin in the midgut of Aedes aegypti L. (Diptera: Culicidae) is separable into two cellular phases. Insect Biochem 21:197–203
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fraser CM (1986) Merck Veterinary Manual 6th edn. Merck, Rahway, New Jersey
Fujikawa K, Ghung DW, Hendrickson LE, Davie EW (1986) Amino acid sequence of human factor XI, a blood coagulation factor with four tandem repeats that are highly homologous with plasma prekallikrein. Biochemistry 25:2417–2424
Gooding RE (1973) The digestive processes of haematophagous insects. IV. Secretion of trypsin by Aedes aegypti. Can I Entomol 105:599–603
Graf R, Briegel H (1985) Isolation of trypsin isozymes from the mosquito Aedes aegypti. Insect Biochem 15:611–618
Graf R, Briegel H (1989) The synthetic pathway of trypsin in the mosquito Aedes aegypti L. (Diptera: Culicidae) and in vitro stimulation in isolated midguts. Insect Biochem 19:129–137
Graf R, Raikhel AS, Brown MR, Lea AO, Briegel H (1986) Mosquito trypsin: immunocytochemical localization in the midgut of blood-fed Aedes aegypti (L). Cell Tissue Res 245:19–27
Grant GA, Henderson KO, Eisen AZ, Bradshaw RA (1980) Amino acid sequence of a collagenolytic protease from the hepatopancreas of the fiddler crab, Uca pugilator. Biochemistry 19:4653–4659
Greer J (1990) Comparative modeling methods: Applications to the family of the mammalian serine proteases. Proteins: Structure, Function and Genetics 7:317–334
Haber E, Quertermous T, Matsueda GR, Runge MS (1989) Innovative approaches to plasminogen activator therapy. Science 243:51–56
Harris TJR (1987) Second generation plasminogen activators. Protein Engineering 1:449–458
Higgins DG, Sharp PM (1989) Fast and sensitive multiple sequence alignments on a microcomputer. Comp Appl Biol Sci 5:151–153
Higgins DL, Bennett WF (1990) Tissue plasminogen activator: the biochemistry and pharmacology of variants produced by mutagenesis. Annu Rev Pharmacol Toxicol 30:91–121
Hogsette JA, Ruff JP (1986) Evaluation of flucythrinate and fenvalerate impregnated eartags and permethrin ear tapes for fly control on beef and dairy cattle in northwest Florida. J Econ Entomol 79:152–157
Houseman JG, Campbell FC, Morrison PE (1987) A preliminary characterisation of digestive proteases in the posterior midgut of the stable fly Stomoxys calcitrans (L.) (Diptera: Muscidae). Insect Biochem 17:213–218
Inoue H, Nojima H, Okayama H (1990) High efficiency transformation of E. coli with plasmids. Gene 96:23–28
Jany K-D, Haug H (1983) Amino acid sequence of the chymotryptic protease II from the larva of the hornet, Vespa crabro. FEBS Lett 158:98–102
Jany K-D, Beckler G, Pleiderer G, Ishay J (1983) Amino acid sequence of an insect chymotrypsin from the larva of the hornet. Biochem Biophys Res Commun 110:1–7
Jin Y, Anderson KV (1990) Dominant and recessive alleles of the Drosophila easter gene are point mutations at conserved sites in the serine protease catalytic domain. Cell 60:873–881
Kemp DH, Agbede RIS, Johnston LAY, Gough JM (1986) Immunization of cattle against Boophilus microplus using extracts derived from adult female ticks: feeding and survival of the parasite on vaccinated cattle. Int J Parasitol 16:115–120
Krätzschmar J, Haendler B, Langer G, Boidol W, Bringmann P, Alagon A, Donner P, Schleuning W-D (1991) The plasminogen activator family from the salivary gland of the vampire bat Desmodus rotundus. cloning and expression. Gene 105:229–237
Kraut JU (1977) Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem 46:331–358
Lecroisey A, Gilles A-M, DeWolf A, Keil B (1987) Complete amino acid sequence of the collagenase from the insect Hypoderma lineatum. J Biol Chem 262:7546–7551
Leytus SP, Loeb KR, Hagen FS, Kurachi K, Davie EW (1988) A novel trypsin-like serine protease hepsin with a putative transmembrane domain expressed by human liver and hepatoma cells. Biochemistry 27:1067–1074
Moffatt MR, Lehane MJ (1990) Trypsin is stored as an inactive zymogen in the midgut of Stomoxys calcitrans. Insect Biochem 20:719–723
Muta T, Hashimoto R, Miyata T, Nishimura H, Toh Y, Iwanaga S (1990) Proclotting enzyme from horseshoe crab haemocytes — cDNA cloning, disulphide locations and subcellular localisation. J Biol Chem 265:22426–22433
Neurath H (1984) Evolution of proteolytic enzymes. Science 224:350–357
Neurath H (1986) The versatility of proteolytic enzymes. J Cellular Biochem 32:35–49
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparisons. Proc Natl Acad Sci (USA) 85:2444–2448
Ross MJ, Grossbard EB, Hotchkiss A, Higgins D, Anderson S (1988) Plasminogen activators. Ann Rep Med Chem 23:111–120
Sakanari JA, Staunton CE, Eakin AE, Craik CS, McKerrow JH (1989) Serine proteases from nematode and protozoan parasites: Isolation of sequence homologues using generic molecular probes. Proc Natl Acad Sci (USA) 86:4863–4867
Sambrook T, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. 2nd edn, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Seddon HR (1967) Diseases of domestic animals in Australia. 2. Arthropod infestations. Serv Publ No6 Dept Health Aust Smith DB, Johnson KS (1988) Single-step purification of polypeptides expressed in E. coli as fusions with glutathione S-transferase. Gene 67: 31-40
Stevenson BT, Hagenbüchle, Wellaner PK (1986) sequence organisation and transcriptional regulation of the mouse elastase II and trypsin genes. Nucleic Acids Res 14:8307–8330
Titani K, Sasagawa T, Woodbury RG, Ericsson LH, Dörsman H, Kraemer M, Neurath H, Zwilling R (1983) Amino acid sequence of crayfish (Astacus fluviatilis) trypsin If. Biochemistry 22:1459–1465
Vaughan JA, Azad AF (1988) Passage of host immunoglobulin G from blood meal into haemolymph of selected mosquito species (Diptera: Culicidae). J Med Entomol 25:472–474
Wikel SK (1988) Immunological control of haematophagous arthropod vectors: utilization of novel antigens. Vet Parasitol 29:235–264
Willadsen P, Kemp DH (1988) Vaccination with ‘concealed antigens’: myth or reality? Parasitol Today 4:196–198
Willadsen P, Riding G, McKenna R, Kemp DH, Tellam R, Nielsen JN, Lahnstein J, Cobon GS, Gough JM (1989) Immunological connrol of a parasitic arthropod: Identification of a protective antigen from Boophilus microplus. J Immunol 143:1346–1351
Woodbury RG, Katunuma N, Kobayashi K, Titani K, Neurath H (1978) Covalent structure of a group-specific protease from rat small intestine. Biochemistry 17: 811–819
Yun Y, Davis RL (1989) Levels of RNA from a family of putative serine protease genes are reduced in Drosophila melanogaster dunce mutants and are regulated by cyclic AMP. Mol Cell Biol 9:692–700
Author information
Authors and Affiliations
Additional information
Communicated by E.K.F. Bautz
Rights and permissions
About this article
Cite this article
Elvin, C.M., Whan, V. & Riddles, P.W. A family of serine protease genes expressed in adult buffalo fly (Haematobia irritans exigua). Molec. Gen. Genet. 240, 132–139 (1993). https://doi.org/10.1007/BF00276892
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00276892