Summary
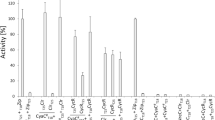
Rates of synthesis of cyclic 3′,5′-adenosine monophosphate (cAMP) were measured in cultures of Escherichia coli aerating without a carbon source. This technique provides a representative measure of adenylate cyclase activity in the absence of inhibition caused by transport of the carbon source. Adenylate cyclase activity was found to vary more than 20-fold depending on the carbon source that had been available during growth. Synthesis of cAMP in cells aerating in the absence of the carbon source was highest when cells had been grown with glucose or fructose which inhibit adenylate cyclase activity severely. Synthesis of cAMP was much lower when cells had been grown with glycerol or succinate which cause only minimal inhibition of the activity.
The variation in cAMP synthesis due to different carbon sources requires a functional cAMP receptor protein (CRP). Crp- mutants synthesize cAMP at comparable rates regardless of the carbon source that afforded growth. A novel mutant of E. coli having a CRP no longer dependent on cAMP has been isolated and characterized. Adenylate cyclase activity in this mutant no longer responds normally to variations in the carbon source.
Similar content being viewed by others
References
Alper, M.D., Ames, B.N.: transport of antibiotics and metabolite analogs by systems under cyclic AMP control: Positive selection of Salmonella typhimurium cya and crp mutants. J. Bact. 133, 149–157 (1978)
Anderson, W.B., Schneider, A.B., Emmer, M., Perlman, R.L., Pastan, I.: Purification and properties of the cyclic adenosine 3′, 5′-monophosphate receptor protein which mediates adenosine 3′,5′-monophosphate dependent gene transcription in Escherichia coli. J. biol. Chem. 245, 5929–5937 (1971)
Bachman, B.J., Low, K.B., Taylor, A.L.: Recalibrated linkage map of Escherichia coli K-12. Bact. Rev. 40, 116–167 (1976)
Braedt, G., Gallant, J.: Role of the rel gene product in the control of cyclic 3′,5′-adenosine monophosphate accumulation. J. Bact. 129, 564–566 (1976)
Brickman, E., Soll, L., Beckwith, J.: Genetic characterization of mutations which affect catabolite sensitive operons in Escherichia coli: Including deletions of the gene for adenyl cyclase. J. Bact. 116, 582–587 (1973)
Buettner, M.J., Spitz, E., Rickenberg, H.V.: Cyclic adenosine 3′,5′-monophosphate in Escherichia coli. J. Bact. 114, 1068–1075 (1973)
Botsford, J.L.: Metabolism of cyclic adenosine 3′,5′-monophosphate and induction of tryptophanase in Escherichia coli. J. Bact. 124, 380–390 (1975)
Dallas, W.S., Tseng, Y.-H., Dobrogosz, W.J.: Regulation of membrane functions and fatty acid composition in Escherichia coli by cyclic AMP receptor protein. Arch. Biochem. Biophys. 175, 295–320 (1976)
Danley, D.E., Drexler, M., Botsford, J.L.: Differential binding of cyclic adenosine 3′,5′-monophosphate to the cyclic adenosine 3′,5′-monophosphate receptor protein in Escherichia coli. J. Bact. 130, 563–565 (1977)
Emmer, M., Crombrugghe, B. de, Pastan, I., Perlman, R.: Cyclic AMP receptor protein of E. coli: Its role in synthesis of inducible enzymes. Proc. nat. Acad. Sci. (Wash.) 60, 480–487 (1970)
Epstein, W., Rothman-Dennes, L.B., Hesse, J.: cAMP as mediator of catabolite repression in Escherichia coli. Proc. nat. Acad. Sci. (Wash.) 72, 2300–2304 (1975)
Gallant, J., Margason, G., Finch, B.: On the turnover of ppGpp in Escherichia coli. J. biol. Chem. 247, 6055–6058 (1972)
Gilman, A.F.: A protein binding assay for cAMP. Proc. nat. Acad. Sci. (Wash.) 67, 305–312 (1970)
Haggerty, D.M., Schleif, R.F.: Kinetics of the onset of catabolite repression in Escherichia coli as determined by lac mRNA iniations and intracellular cAMP levels. J. Bact. 123, 946–953 (1975)
Harwood, J.P., Gazdar, C., Prasad, C., Curtis, S.J., Epstein W., Peterkofsky, A.: Involvement of the glucose enzymes II of the sugar phosphotransferase in the regulation of adenylate cyclase in Escherichia coli. J. biol. Chem. 251, 2462–2468 (1976)
Harwood, J., Peterkofsky, A.: Glucose sensitive adenylate cyclase in toluene treated cells of Escherichia coli. J. biol. Chem. 250, 4656–4662 (1975)
Hua, S.-S., Markovitz, A.: Regulation of the galactose operon at the gal operator-promoter in Escherichia coli K-12. J. Bact. 122, 510–517 (1975)
Kaplan, S., Atherly, A.G., Barreit, A.: Synthesis of stable RNA in stringent E. coli in the absence of charged tRNA. Proc. nat. Acad. Sci. (Wash.) 70, 683–692 (1973)
Kumar, S.: Properties of cya and crp mutants of Escherichia coli. J. Bact. 125, 545–555 (1976)
Leavitt, R.I., Umbarger, H.E.: Isoleucine and valine metabolsim in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli K-12. J. Bact. 83, 624–630 (1962)
Magasanik, B.: Inducer exclusion and repression, pp. 219–241. In: The lac operon. (D. Zipser and J. R. Beckwith, eds.), New York: Cold Spring Harbor Laboratory 1970.
Miller, J.H.: Experiments in molecular gentics. New York: Cold Spring Harbor Laboratory 1972
Nielsen, L.D., Monard, D., Rickenberg, H.V.: Cyclic 3′,5′-adenosine monophosphate phosphodiesterase of Escherichia coli. J. Bact. 116, 857–866 (1973)
Pastan, I., Adhya, S.: Cyclic adenosine 3′,5′-monophosphate in Escherichia coli. Bact. Rev. 40, 527–551 (1976)
Pastan, I., Galli, M., Anderson, W.B.: The purification and analysis of mechanism of action of a cyclic AMP-receptor protein from Escherichia coli. Meth. in Enz. 38 (Part C), 367–375 (1974)
Peterkofsky, A.: Regulation of Escherichia coli adenylate cyclase by phosphorylation-dephosphorylation. Trends in Biochem. Sci. 2, 2–14 (1977)
Peterkofsky, A., Gazdar, C.: Glucose inhibition of adenylate cyclase in intact cells of Escherichia coli B. Proc. nat. Acad. Sci. (Wash.) 71, 2324–2378 (1974)
Peterkofsky, A., Gazdar, C.: Interaction of enzyme I of the phosphoenol pyruvate sugar phosphotransferase system with adenylate cyclase. Proc. nat. Acad. Sci (Wash.) 72, 2920–2924 (1975)
Peterkofsky, A., Gazdar, C.: Glucose and the metabolism of adenosine 3′:5′-cyclic monophosphate in Escherichia coli. Proc. nat. Acad. Sci. (Wash.) 68, 2794–2798 (1971)
Peterkofsky, A., Gazdar, C.: Measurements of cAMP synthesis in intact Escherichia coli B. Proc. nat. Acad. Sci. (Wash.) 70, 2143–2152 (1973)
Peterkofsky, A., Harwood, J., Gazdar, C.: Inducibility of sugar sensitivity of adenylate cyclase of E. coli B. J. Cyclic Nucl. Res. 1, 11–20 (1975)
Potter, K., Chalmers-Larson, G., Yamazaki, H.: Abnormally high rate of cyclic AMP excretion from an Escherichia coli mutant deficient in cyclic AMP receptor protein. Biochem. biophys. Res. Commun. 57, 379–386 (1974)
Prusiner, S., Miller, R.W., Valentine, R.C.: Adenosine 3′:5′-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc. nat. Acad. Sci. (Wash.) 69, 2922–2926 (1973)
Rephaeli, A.W., Saier, M.H.: Effects of crp mutations on adenosine 3′:5′-monophosphate metabolism in Salmonella typhimurium. J. Bact. 127, 120–127 (1976)
Saier, M.H.: Bacterial phosphoenolpyruvate sugar phosphotransferase systems: Structural, functional, and evolutionary interrelationship. Bact. Rev. 41, 856–871 (1977)
Saier, M.H., Feucht, B.U., Hofstadler, L.H.: Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzyme II of the phosphoenol pyruvate: sugar phosphotransferase system in Escherichia coli. J. biol. Chem. 251, 883–892 (1976)
Saier, M.H., Feucht, B.U.: Co-ordinate regulation of adenylate cyclase and carbohydrate transport by phosphoenol pyruvate sugar phosphotransferase system in Salmonella typhimurium. J. biol. Chem. 250, 7078–7080 (1975)
Saier, M.H., Feucht, M.H., McCamen, R.T.: Regulation of intracellular adenosine 3′:5′-monophosphate levels in Escherichia coli and Salmonella typhimurium. J. biol. Chem. 250, 7593–7597 (1975)
Sanders, R., McGeoch, D.: A mutant transcription factor that is activated by 3′,5′-cyclic guanosine monophosphate. Proc. nat. Acad. Sci. (wash.) 70, 1077–1081
Silverstone, A.G., Groman, M., Scaife, J.G.: A1 +: a new factor involved in the synthesis of RNA by Escherichia coli. Molec. gen. Genet. 118, 223–234 (1972)
Wayne, P.K., Rosen, O.M.: Cyclic 3′,5′-adenosine monophosphate in Escherichia coli during transient and catabolite repression. Pro. nat. Acad. Sci. (Wash.) 71, 1436–1440 (1974)
Zubay, G., Schwartz, D., Beckwith, J.: Mechanism of activation of catabolite sensitive genes: A positive control system. Proc. nat. Acad. Sci. (Wash.) 60, 104–110 (1970)
Author information
Authors and Affiliations
Additional information
Communicated by G. O'Donovan
Rights and permissions
About this article
Cite this article
Botsford, J.L., Drexler, M. The cyclic 3′,5′-adenosine monophosphate receptor protein and regulation of cyclic 3′,5′-adenosine monophosphate synthesis in Escherichia coli . Molec. Gen. Genet. 165, 47–56 (1978). https://doi.org/10.1007/BF00270375
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00270375