Summary
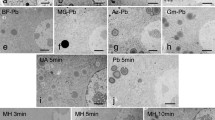
A method is presented for increasing the contrast of cellular structures on ultrathin sections from tissues embedded in Lowicryl K4M. The method, designated UA/MC adsorption staining, is based on the uranyl acetate/methyl cellulose staining of thawed cryosections. Ultrathin Lowicryl K4M sections were exposed to a uranyl acetate/methyl cellulose solution and the excess solution was removed with filter paper, leaving the remainder to air dry on the section. Sections on the grids were then directly observed in the electron microscope. Parameters such as methyl cellulose and uranyl acetate concentrations, duration of staining, temperature and pH were all assessed for their effect on subsequent contrast formation. Conditions were achieved which yielded intense contrast of cellular membranes, basement membranes and extracellular matrix components usually not apparent in Lowicryl K4M thin sections routinely counter-stained with uranyl acetate and lead acetate. The enhancement of the contrast of these structures does not obscure colloidal gold particles used for immunocytochemistry or lectin labeling, thus making the UA/MC adsorption staining method useful for increasing membrane contrast in routine post-embedding immuno- and lectin cytochemistry on Lowicryl K4M thin sections.
Similar content being viewed by others
References
Acetarin J-D, Carlemalm E, Kellenberger E, Villiger W (1987) Correlation of some mechanical properties of embedding resins with their behaviour in microtomy. J Electron Microsc Tech 6:63–79
Armbruster BL, Garavito RM, Kellenberger E (1983) Dehydration and embedding temperatures affect the antigenic specificity of tubulin and immunolabeling by the protein A-colloidal gold technique. J Histochem Cytochem 31:1380–1384
Bendayan M, Zollinger M (1983) Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem 31:101–109
Benichou JC, Frehel C, Ryter A (1990) Improved sectioning and ultrastructure of bacteria and animal cells embedded in Lowicryl. J Electron Microsc Tech 14:289–297
Berryman MA, Rodewald RD (1990) An enhanced method for post-embedding immunocytochemical staining which preserves cell membranes. J Histochem Cytochem 38:159–170
Brada D, Kerjaschki D, Roth J (1990) Cell type-specific post-Golgi apparatus localization of a “resident” endoplasmic reticulum glycoprotein, glucosidase II. J Cell Biol 110:309–318
Carlemalm E, Garavito RM, Villiger W (1982) Resin development for electron microscopy and an analysis of embedding at low temperature. J Microsc 126:123–143
Carlemalm E, Colliex C, Kellenberger E (1985) Contrast formation in electron microscopy of biological material. In: Hawkes PW (ed) Advances in electronics and electron physics, vol. 63. Academic Press, New York, pp 269–334
Egea G, Goldstein IJ, Roth J (1989) Light and electron microscopic detection of (3 Gal β1,4 GlcNAc β1) sequences in asparagine-linked oligosaccharides with the Datura stramonium lectin. Histochemistry 92:515–522
Griffiths G, McDowall A, Back R, Dubochet J (1984) On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res 89:65–78
Hauri H-P, Roth J, Sterchi EE, Lentze MJ (1985) Transport to cell surface of intestinal sucrase-isomaltase is blocked in the Golgi apparatus in a patient with congenital sucrase-isomaltase deficiency. Proc Natl Acad Sci USA 82:4423–4427
Hayat MA (1981) Principles and techniques of electron microscopy. Biological applications, vol. 1. University Park Press, Baltimore, MD
Kanwar YS, Farquhar MG (1979) Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol 81:137–153
Kellenberger E, Villiger W, Carlemalm E (1986) The influence of the surface relief of thin sections of embedded, unstained biological material on image quality. Micron Microsc Acta 17:331–348
Kellenberger E, Durenberger M, Villiger W, Carlemalm E, Wurtz M (1987) The efficiency of immunolabel on Lowicryl sections compared to theoretical predictions. J Histochem Cytochem 35:959–969
Larrson L-I (1988) Immunocytochemistry: theory and practice. CRC Press, Boca Raton, FL, USA
Lucocq JM, Baschong W (1986) Preparation of protein colloidal gold complexes in the presence of commonly used buffers. Eur J Cell Biol 42:332–337
Millonig G (1961) A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol 11:736–739
Nakane PK, Pierce GB (1966) Enzyme-labeled antibodies: preparation and application for the localization of antigens. J Histochem Cytochem 14:929–931
Roth J (1983a) The colloidal gold marker system for light and electron microscopic cytochemistry. In: Bullock GR, Petrusz P (eds) Techniques in immunocytochemistry, vol. 2. Academic Press, London, pp 217–284
Roth J (1983b) Application of lectin-gold complexes for electron microscopic localization of glycoconjugates on thin sections. J Histochem Cytochem 31:987–999
Roth J (1984) Cytochemical localization of terminal N-acetyl-d-galactosamine residues in cellular compartments of intestinal goblet cells: Implications for the topology of O-glycosylation. J Cell Biol 98:399–406
Roth J (1986) Post-embedding cytochemistry with gold-labeled reagents: a review. J Microsc 143:125–137
Roth J (1987) Light- and electron microscopic localization of glycoconjugates with gold-labeled reagents. Scanning Microsc 1:695–704
Roth J (1989) Postembedding labeling on Lowicryl K4M tissue sections: detection and modification of cellular components. Methods Cell Biol 31:513–551
Roth J, Berger EG (1982) Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol 93:223–229
Roth J, Bendayan M, Orci L (1978) Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem 26:1074–1081
Roth J, Bendayan M, Carlemalm E, Villiger W, Garavito M (1981) Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem 29:663–671
Roth J, Brown D, Orci L (1983) Regional distribution of N-acetyl-d-galactosamine residues in the glycocalyx of glomerular podocytes. J Cell Biol 96:1189–1196
Roth J, Lucocq J, Charest PM (1984) Light- and electron microscopic demonstration of sialic acid residues with the lectin from Limax flavus: a cytochemical affinity technique with the use of fetuin-gold complexes. J Histochem Cytochem 32:1167–1176
Roth J, Lentze MJ, Berger EG (1985a) Immunocytochemical demonstration of ecto-galactosyltransferase in absorptive intestinal cells. J Cell Biol 100:118–125
Roth J, Taatjes DJ, Lucocq JM, Weinstein J, Paulson JC (1985b) Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus cisternal stack that may function in glycosylation. Cell 43:287–295
Roth J, Taatjes DJ, Weinstein J, Paulson JC, Greenwell P, Watkins WM (1986) Differential subcompartmentation of terminal glycosylation in the Golgi apparatus of intestinal absorptive and goblet cells. J Biol Chem 261:14307–14312
Tokuyasu KT (1978) A study of positive staining of ultrathin frozen sections. J Ultrastruct Res 63:287–307
Tokuyasu KT (1980a) Immunochemistry on ultrathin frozen sections. Histochem J 12:381–403
Tokuyasu KT (1980b) Adsorption staining method for ultrathin frozen sections. In: Bailey GW (ed) Proceedings of the 38th Meeting of the Electron Microscopy Society of America. Claitor, Baton Rouge, pp 760–763
Tokuyasu KT (1989) Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J 21:163–171
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roth, J., Taatjes, D.J. & Tokuyasu, K.T. Contrasting of Lowicryl K4M thin sections. Histochemistry 95, 123–136 (1990). https://doi.org/10.1007/BF00266584
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00266584