Summary
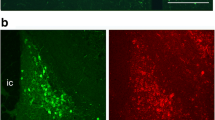
Immunocytochemical light and electron microscopic studies revealed two distinct populations of corticotropin releasing factor (CRF) — containing neurons, a dorsolateral and ventrolateral group, located in the bed nucleus of the stria terminalis (BST) of the rat brain. CRF neurons of the dorsolateral group had a smaller diameter and more primary dendrites than those of the ventrolateral group. CRF neurons in the dorsolateral BST had both somatic and dendritic spines, smooth contoured nuclei, and many dense and alveolate vesicles in their cytoplasm. Whereas, CRF neurons in the ventrolateral BST had only dendritic spines, irregularly-shaped indented nuclei and contained only alveolate vesicles in their cytoplasm.
The only obvious difference in the type of unidentified afferents that synapsed on the CRF neurons of the BST could be attributed to the presence of the somatic spines on the CRF neurons of the dorsolateral population. Otherwise, the CRF neurons of the BST had a profuse innervation that included axosomatic, axospinous and axodendritic synapses.
CRF-containing axons were distributed unevenly throughout the BST. The density of CRF axons was greatest in the lateral subdivisions of the BST, but the ventromedial BST contained many more CRF axons than the dorsomedial BST.
The presence of these two CRF neuron populations in the BST suggests functional subdivision beyond previous proposals of a medial and lateral separation of function. Now there is additional morphological evidence to support the proposal of a dorsal and ventral separation of function within the BST.
Similar content being viewed by others
References
Albert DJ, Walsh ML (1982) The inhibitory modulation of agonistic behavior in the rat brain: a review. Neurosci Biobehav Rev 6:125–143
Allen TO, Adler NT, Greenberg JH, Reivich M (1981) Vaginocervical stimulation selectively increases metabolic activity in the rat brain. Science 211:1070–1072
Beltramino C, Taleisnik S (1980) Dual action of electrochemical stimulation of the bed nucleus of the stria terminalis on the release of LH. Neuroendocrinology 30:238–242
Berod A, Hartman BK, Pujol JF (1981) Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem 29:844–850
Brown M (1986) Corticotropin releasing factor: central nervous system sites of action. Brain Res 399:10–14
Brown MR, Fisher L (1985) Corticotropin-releasing factor: effects on the autonomic nervous system and visceral systems. Fed Proc 44:243–248
Brown M, Gray T, Fisher L (1986) Corticotropin-releasing factor receptor antagonist: effects on the autonomic nervous system and cardiovascular function. Regul Pept 16:321–329
Bueno J, Pfaff DW (1976) Single unit recording in hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rats. Brain Res 101:67–78
Canonaco M, O'Connor LH, Pfaff DW, McEwen BS (1989) Longer-term progesterone treatment induces changes of GABAa receptor levels in forebrain sites in the female hamster: quantitative autoradiography study. Exp Brain Res 77:407–411
Cassell MD, Gray TS (1989) The amygdala directly innervates adrenergic (C1) neurons in the ventrolateral medulla in the rat. Neurosci Lett 97:163–168
Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB (1986) Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain areas after acute and chronic stress. J Neurosci 6:2908–2914
Cintra A, Fuxe K, Harfstrand A, Agnati L, Wikstrom A, Okret S, Vale W, Gustafsson J (1987) Presence of glucocorticoid receptor immunoreactivity in corticotrophin releasing factor immunoreactive neurons in the rat di- and telencephalon. Neurosci Lett 77:25–30
Coss RG, Perkel DH (1985) The function of dendritic spines: A review of theoretical issues. Behav Neural Biol 44:151–185
Cummings S, Elde R, Ells J, Lindall A (1983) Corticotropin releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci 3:1355–1368
Dalsass M, Seigel A (1987) Electrophysiological examination of neurons in the bed nucleus of the stria terminalis: Characteristic properties and responses to amygdaloid and hypothalamic stimulation. Brain Res 542:357–365
de Olmos J, Alheid GF, Belatramino CA (1985) Amygdala. In: G Paxinos (ed) The rat nervous system, vol 1. Academic Press, New York, pp 251–269
Dunn JD (1987) Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res 407:327–331
Eaves M, Thatcher-Britton K, Rivier J, Vale W, Koob GF (1985) Effects of corticotropin releasing factor on locomotor activity in hypophysectomized rats. Peptides 6:923–926
Eberly LB, Dudley CA, Moss RL (1983) Iontophoretic mapping of corticotropin-releasing factor (CRF) sensitive neurons in the rat forebrain. Peptides 4:837–841
Ehlers CL (1986) EEG stability following corticotropin releasing factor in rats. Psychoneuroendocrinol 11:121–125
Emery DE, Sachs (1976) Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol Behav 17:803–806
Faiers AA, Calaresu FR, Mogenson GJ (1976) Factors affecting cardiovascular responses to stimulation of hypothalamus in the rat. Exp Neurol 51:188–206
Freund TF, Powell JF, Smith AD (1984) Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience 13:1189–1215
Gainer H, Russell JT, Loh YP (1985) The enzymology and intracellular organization of peptide precursor processing: the secretory vesicle hypothesis. Neuroendocrinology 40:171–184
Gray TS (1986) Neuropeptide neuronal efferents from the amygdala to the central gray in the rat. Anatom Rec 214:44
Haas DA, George SR (1989) Estradiol or ovariectomy decreases CRF synthesis in hypothalamus. Br Res Bull 23:215–218
Henke GP (1984) The bed nucleus of the stria terminalis and immobilization-stress: Unit activity, escape behaviour, and gastric pathology in rats. Behav Brain Res 11:35–45
Holstege G, Meiners L, Tan T (1985) Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res 58:379–391
Itaya SK, Van Hoesen GW, Jenq C-B (1981) Direct retinal input to the limbic system of the rat. Brain Res 226:33–42
Kawakami M, Kimura F (1976) Limbic-preoptic responses to estrogens and catecholamines in relation to cyclic LH secretion. In: Naftolin F, Ryan KJ, Davies J (eds) Subcellular mechanisms in reproductive neuroendocrinology. Elsevier, Amsterdam, pp 423–452
Kawakami M, Terasawa E (1974) Role of limbic forebrain structures on reproductive cycles. In: Kawakami M (ed) Biological rhythms in neuroendocrine activity. Igaku Shoin, Tokyo, pp 197–219
Lenz HJ, Raedler A, Greten H, Brown MR (1987) CRF initiates biological actions within the brain that are observed in response to stress. Am J Physiol 252:R34-R39
Leranth C, Antoni FA, Palkovits M (1983) Ultrastructural demonstration of ovine CRF-like immunoreactivity (oCRF-LI) in the rat hypothalamus: processes of magnocellular neurons establish membrane specializations with parvocellular neurons containing oCRF-LI. Regul Pept 6:179–188
Leranth C, MacKlusky NJ, Naftolin F (1986) Interconnections between neurotransmitter- and neuropeptide-containing neurons involved in gonadotrophin release in the rat. In: Moody TW (ed) Neural and endocrine peptides and receptors. Plenum Press, New York, pp 177–193
Lichtensteiger W (1974) Extrahypothalamic influences on the tuberoinfundibular dopamine neurones and the secretion of luteinizing hormone (LH) and prolactin. In: Knowles F, Vollrath L (eds) Neurosecretion — The final neuroendocrine pathway. Springer, Berlin Heidelberg New York, pp 229–240
Lindsey JD, Ellisman MH (1985) The neuronal endomembrane system. I. Direct links between rough endoplasmic reticulum and the cis element of the Golgi apparatus. J Neurosci 5:3111–3123
Liposits Zs, Gorcs T, Setalo G, Lengvari I, Flerko B, Vigh S, Schally AV (1983) Ultrastructural characteristics of immunolabeled, corticotropin-releasing factor (CRF)-synthesizing neurons in the rat brain. Cell Tiss Res 229:191–196
Liposits Zs, Paull WK (1985a) Ultrastructural alteration of the paraventriculo-infundibular corticotropin releasing (CRF) — immunoreactive neuronal system in long-term adrenalectomized rats. Peptides 6:1021–1036
Liposits Zs, Paull WK, Setalo G, Vigh S (1985b) Evidence for local corticotropin releasing factor (CRF)-immunoreactive neuronal circuits in the paraventricular nucleus of the rat hypothalamus. An electron microscopic immunohistochemical analysis. Histochemistry 83:5–16
Liposits Zs, Phelix C, Paull WK (1986a) Adrenergic innervation of corticotropin-releasing factor (CRF)-synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A combined light and electron microscopic immunocytochemical study. Histochemistry 84:201–205
Liposits Zs, Sherman D, Phelix C, Paull WK (1986b) A combined light and electron microscopic immunocytochemical method for the simultaneous localization of multiple tissue antigens. Tyrosine hydroxylase immunoreactive innervation of corticotropin releasing factor synthesizing neurons in the paraventricular nucleus of the rat. Histochemistry 85:95–106
McDonald AJ (1983) Neurons in the bed nucleus of the stria terminalis: A golgi study in the rat. Brain Res Bull 10:111–120
Merchenthaler I, Vigh S, Petrusz P, Schally AV (1982) Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. Am J Anat 165:385–396
Merchanthaler I, Vigh S, Petrusz P, Schally AV (1983) The paraventriculo-infundibular corticotropin-releasing factor (CRF) pathway as revealed by immunocytochemistry in long-term hypophysectomized or adrenalectomized rats. Regul Pept 5:295–305
Moga MM, Gray T (1985) Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J Comp Neurol 241:275–284
Moga MM, Gray T, Saper CB (1987) Subnuclear organization of the bed nucleus of the stria terminalis: a cytoarchitectural, connectional and immunocytochemical study. Neurosci Abs 13:443
Moga MM, Saper CB, Gray TS (1989) Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry and projection to the parabrachial nucleus in the rat. J Comp Neurol 283:315–332
Moore RY (1978) Catecholamine innervation of the basal forebrain. I. The septal area. J Comp Neurol 177:665–684
Nikolarakis KE, Almeida OFX, Herz A (1986a) Corticotropin-releasing factor inhibits gonadotropin-releasing hormone (GnRH) release from superfused rat hypothalami in-vitro. Brain Res 377:388–390
Nikolarakis KE, Almeida OFX, Herz A (1986b) Stimulation of hypothalamic beta-endorphin and dynorphin release by corticotropin-releasing factor (in vitro). Brain Res 399:152–155
Oades RD (1985) The role of noradrenaline in tuning and dopamine in switching between signals in the CNS. Neurosci Biobehav Res 9:261–282
Olshowka JA, O'Donohue TL, Mueller GP, Jacobowitz DM (1982) The distribution of corticotropin releasing factor-like immuno-reactive neurons in rat brain. Peptides 3:995–1015
Ono N, Bedran-DeCastro JC, McCann SM (1985) Ultrashort-loop feedback of corticotropin (ACTH)-releasing factor to enhance ACTH release in stress. Proc Natl Acad Sci USA 82:3528–3531
Pfaff D, Keiner M (1973) Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol 151:121–158
Pickel VM (1985) General morphological features of peptidergic neurons. In: Bjorklund A, Hokfelt T (eds) Handbook of chemical neuroanatomy, vol 4. GABA and neuropeptides in the CNS, part I. Elsevier, Amsterdam, pp 72–92
Rivier C, Vale W (1984) Influence of corticotropin-releasing factor on reproductive functions in the rat. Endocrinol 114:914–921
Rivier C, Rivier J, Vale W L (1986) Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science 231:607–609
Sakanaka M, Magari S, Sibasaki T, Inoue N (1989) Co-localization of corticotropin-releasing factor- and enkephalin-like immunoreactivities in nerve cells of the rat hypothalamus and adjacent areas. Br Res 487:357–362
Sakanaka M, Shibasaki T, Lederis K (1986a) Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res 382:213–238
Saphier D, Feldman S (1986) Electrophysiology of limbic forebrain and paraventricular nucleus connections. Brain Res Bull 17:743–750
Sawada S, Yamamoto C (1981) Postsynaptic inhibitory actions of catecholamines and opioid peptides in the bed nucleus of the stria terminalis. Exp Brain Res 41:264–270
Sawchenko PE (1987) Adrenalectomy-induced enhancement of CRF and vasopressin immunoreactivity in parvocellular neurosecretory neurons: anatomic, peptide, and steroid specificity. J Neurosci 7:1093–1106
Sawchenko PE, Swanson LW (1983) The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol 218:121–144
Sawchenko PE, Swanson LW (1985) Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Fed Proc 44:221–228
Shaikh MB, Brutus M, Siegel HE, Siegel A (1985) Forebrain structures regulating flight behavior in the cat. Brain Res Bull 14:217–221
Shaikh MB, Brutus M, Siegel HE, Siegel A (1986) Regulation of feline aggression by the bed nucleus of the stria terminalis. Brain Res Bull 16:179–182
Sheridan PJ (1979) The nucleus interstitialis striae terminalis and the nucleus amygdaloideus medialis: prime targets for androgen in the rat forebrain. Endocrinology 104:130–136
Sherman JE, Kalin NH (1986) Corticotrophin-releasing hormone effects on stress-related behavior in rats. In: Terry E (ed) Neural and endocrine peptides and receptors. Plenum Press, New York, pp 195–204
Sherman JE, Kalin NH (1987) The effects of ICV-CRH on novelty-induced behavior. Pharmacol Biochem Behav 26:694–703
Siegel J, Morton C, Sandkuhler J, Xiao H, Zimmerman M (1986) Spinal neuronal inhibition and EEG synchrony by electrical stimulation in subcortical forebrain regions of the cat. Exp Brain Res 62:363–372
Siggins GR, Gruol DL (1986) Mechanisms of transmitter action in the vertebrate central nervous system. In: Handbook of physiology, section 1. The nervous system. Mountcastle VB, Bloom FE, Geiger SR (eds) Waverly Press, Maryland, pp 1–114
Silverman AJ (1987) Ultrastructural evidence for corticotropin releasing hormone (CRH) synapses within the paraventricular nucleus of the hypothalamus (PVH) of the rat. Neurosci Abst 13:197
Sirinathsinghji D (1985) Modulation of lordosis behaviour in female rat by corticotropin-releasing factor, beta-endorphin and gonadotropin-releasing hormone in the mesencephalic central gray. Brain Res 339:45–55
Sirinathsinghji DJS, Rees LH, Rivier J, Vale W (1983) Corticotropin-releasing factor is a potent inhibitor of sexual receptivity in the female rat. Nature 305:232–235
Skofitsch G, Jacobowitz DM (1985) Distribution of corticotropin releasing factor-like immunoreactivity in the rat brain by immunohistochemistry and radioimmunoassay: comparison of ovine and rat/human antisera. Peptides 6:319–336
Sofroniew MV (1983) Direct reciprocal connections between the bed nucleus of the stria terminalis and dorsomedial medulla oblongata: evidence from immunohistochemical detection of tracer proteins. J Comp Neurol 213:399–405
Swanson LW (1986) Organization of mammalian neuroendocrine system. In: Mountcastle VB, Bloom FE, Geiger SR (eds) Handbook of physiology, section 1. The nervous system, vol 4. Waverly Press, Maryland
Swanson LW, Cowan WM (1979) The connections of the septal region in the rat. J Comp Neurol 186:621–656
Swanson LW, Hartman BK (1975) The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol 163:467–506
Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186
Swerdlow NR, Swanson LW, Koob GF (1984) Substantia innominata: a critical link in the behavioral expression of mesolimbic dopamine stimulation in the rat. Neurosci Lett 50:19–24
Takahashi LK, Lisk RD (1985) Diencephalic sites of progestin action for inhibiting aggression and facilitating sexual receptivity in estrogen-primed golden hamsters. Endocrinology 116:2393–2399
Tazi A, Dantzer R, Le Moal M, Rivier J, Vale W, Koob GF (1987a) Corticotropin-releasing factor antagonist blocks stress-induced fighting in rats. Regul Pept 18:37–42
Tazi A, Swerdlow NR, Le Moal M, Rivier J, Vale W, Koob GF (1987b) Behavioral activation by CRF: evidence for the involvement of the ventral forebrain. Life Sci 41:41–49
Turner BH, Knapp ME (1976) Projections of the nucleus and tracts of the stria terminalis following lesions at the level of the anterior commissure. Exp Neurol 51:468–479
Vigh S, Merchenthaler I, Torres-Aleman I, Sueiras-Diaz, Coy DV, Carter WH, Petrusz P, Schally AV (1982) Corticotropin-releasing factor (CRF): immunocytochemical localization and radioimmunoassay (RIA). Life Sci 31:2441–2448
Wallace DM, Magnuson DJ, Gray TS (1989) The amygdalo-brain-stem pathway: selective innervation of dopaminergic, nordrenergic and adrenergic cells in the rat. Neurosci Lett 97:252–258
Watson RE, Troiano R, Poulakos J, Weiner S, Block C, Siegel A (1983) Deoxyglucose analysis of the functional neural pathways of the limbic forebrain in the rat. I. The amygdala. Brain Res Rev 5:1–44
Weller KL, Smith DA (1982) Afferent connections to the bed nucleus of the stria terminalis. Brain Res 232:255–270
Author information
Authors and Affiliations
Additional information
Supported by NIH Grant NS19266
Rights and permissions
About this article
Cite this article
Phelix, C.F., Paull, W.K. Demonstration of distinct corticotropin releasing factor — containing neuron populations in the bed nucleus of the stria terminalis. A light and electron microscopic immunocytochemical study in the rat. Histochemistry 94, 345–364 (1990). https://doi.org/10.1007/BF00266441
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00266441