Summary
Midazolam is a water soluble benzodiazepine, with a short elimination half-life in adults and children. An IV bolus (0.2 mg·kg−1) immediately followed by continuous infusion of 0.06 mg·kg−1·h−1 was administered to 15 critically ill neonates at a gestational age of 32.8 weeks, who required sedation for mechanical ventilation. Heart rate and blood pressure were closely monitored.
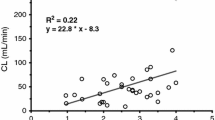
Hypotension occurred in 4 patients after the bolus dose or during the continuous infusion. Three of them had also been given fentanyl. Individual pharmacokinetic parameters were calculated: plasma clearance was 3.9 ml·min−1, elimination half-life was 12.0 h. Because of its short half-life compared to diazepam, midazolam may be used during the neonatal period to achieve rapid, brief sedation. However, it should be administered cautiously to neonates, particularly in premature infants, or if fentanyl is also given.
Similar content being viewed by others
References
Farina ML, Levati A, Tognoni G (1981) A multi-center study of intensive care drug utilisation. Intensive Care Med 7: 125–131
Morselli PL, Principi N, Tognoni G, Reali E, Belvedere G, Standen S, Sereni F (1973) Diazepam elimination in premature and full-term infants and children. J Perinat Med 1: 133–141
Olive G, Rey E (1982) Pharmacocinétique comparée des benzodiazépines. Nouv Presse Med 11: 2957–2964
Reves JG, Fragen R, Vinik HR, Greenblatt DJ (1985) Midazolam: pharmacology and uses. Anesthesiology 62: 310–324
Saint-Maurice C, Meistelman C, Rey E, Esteve C, De Lauture D, Olive G (1986) The pharmacokinetics of rectal midazolam for premedication in children. Anesthesiology 65: 536–538
Jacqz-Aigrain E, Wood E, Robieux I (1990) Pharmacokinetics of midazolam in critically ill neonates. Eur J Clin Pharmacol 36: 191–192
Booker PD, Beechey A, Lloyd-Thomas AR (1986) Sedation of children requiring ventilation using an infusion of midazolam. Br J Anaesth 58: 1104–1108
Puglisi CV, Pao J, Ferrara FJ, de Silva JAF (1985) Determination of midazolam (VersedR) and its metabolites in plasma by high-performance liquid chromatography. J Chromatogr 344: 199–209
Anand KJS, Hickey PR (1987) Pain and its effects in the human neonate and foetus. N Engl J Med 9: 1321–1329
Allonen H, Ziegler G, Klotz U (1981) Midazolam kinetics. Clin Pharmacol Ther 30: 653–661
Rosen DA, Rosen KR (1990) Pain and sedation in the paediatric patient: the 1990 approach to a very old problem. In: Vincent JL (eds) 10th update in intensive care and emergency medecine. Spinger, Berlin Heidelberg New York
Heikkilä J, Arola M, Kanto J, Laaksonen V (1984) Midazolam as adjunct to high dose fentanyl anaesthesia for coronary artery bypass grafting operation. Acta Anaesthesiol Scand 28: 683–689
Koehntop DE, Rodman JH, Brundage DM, Hegland MG, Buckley JJ (1986) Pharmacokinetics of fentanyl in neonates. Anesth Analg 65: 227–232
Burtin P, Daoud P, Jacqz-Aigrain E, Mussat P, Moriette G (1991) Hypotension with midazolam and fentanyl in the newborn. Lancet 337: 1545–1546
Payne K, Matteyse FJ, Liebenberg D, Dawes T (1989) The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol 37: 267–272
Hartwig S, Roth B, Theisohn M (1990) Disposition and sedative effects of midazolam after continuous infusion in artificially ventilated newborns and infants. Abstract. European Society for developmental Pharmacology. Tremezzo, Como (Italy)
Krombach T, Mathys D, Umeno M, Gonzales FJ, Meyer UA (1989) Oxidation of midazolam and triazolam by human cytochrome P450IIIA4. Mol Pharmacol 36: 89–96
Cresteil T, Beaune P, Kremers P, Celier C, Guengerich FP, Leroux JP (1985) Immunoquantification of epoxide hydrolases and cytochrome P-450 isozymes in fetal and adult human liver microsomes. Eur J Biochem 151: 345–350
Guignard JP, Torrado A, Da Cunha O, Gautier F (1975) Glomerular filtration rate in the first weeks of life. J Paediatr 87: 268–272
Morselli PL, Franco-Morselli R, Bossi L (1980) Clinical pharmacokinetics in newborns and infants: age-related differences and clinical implications. Clin Pharmacokinet 5: 485–527
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jacqz-Aigrain, E., Daoud, P., Burtin, P. et al. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. Eur J Clin Pharmacol 42, 329–332 (1992). https://doi.org/10.1007/BF00266357
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00266357