Summary
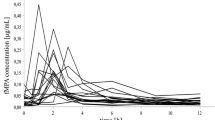
Cyclosporin A (CsA) pharmacokinetics was studied in 19 diabetic children (mean age: 10.6 y). They were divided into prepubertal (I) and pubertal (II) groups according to plasma oestradiol or testosterone concentrations. The kinetic study was performed after a 72 h wash out period and a single oral dose of 7.5 mg/kg CsA. CsA in blood was measured by HPLC. The kinetic parameters: Cmax, tmax, t1/2, AUC, CL/f, Vz/f and tss were calculated.
No significant difference was found between the two groups. A significant negative correlation was found between Vz and both total cholesterol (r=0.46), VLDL+LDL−cholesterol (r=−0.49) and VLDL+LDL−phospholipids (r=−0.58). CsA kinetics at steady-state were simulated by superimposition of single dose kinetics derived from each single dose. Measured steady-state blood concentrations were correlated (r=0.80) with the values predicted by the simulation. The results suggest that CsA adjustment dosage of the CsA may be performed after a single oral dose using blood levels measured by HPLC. This procedure requires validation in further studies.
Similar content being viewed by others

References
d'Athis P, Dusserre L (1983) Déterminer le temps d'accès à l'équilibre lors d'une administration répétée de médicament. J Suiv Ther 1: 45–47
d'Athis P (1988) Programme TRIOMPHE. Traitement intéractif ordonné des modèles pharmacologiques élémentaires Département d'informatique médicale. Faculté de médecine. CHUR, Dijon, France
Assan R, Feutren G, Debray-Sachs M, Quinion-Debrie MC, Laborie C, Thomas G, Chatenoud L, Bach JF (1985) Metabolic and immunological effects of cyclosporin in recently diagnosed type 1 Diabetes Mellitus. Lancet I: 67–71
Beveridge T, Gratwohl A, Michot F, Niederberger W, Nuesch E, Nussbaumer K, Schaub P, Speak B (1981) Cyclosporine A: pharmacokinetics after a single dose in man and serum levels after multiple dosing in recipients of allogeneic bone-marrow grafts. Curr Ther Res 30: 5–18
Beveridge T (1986a) Clinical transplantation-overview. Prog Allergy 38: 269–292
Beveridge T (1986b) Interim report of clinical studies. Prog Allergy 38: 436–446
Bougneres PF, Carel JC, Castano L, Boitard C, Gardin JP, Landais P, Hors J, Mihatsch MJ, Paillard M, Chaussain JL, Bach JF (1988) Factors associated with early remission of type I diabetes in children treated with cyclosporin. N Engl J Med 318: 663–670
Burckart JG, Venkataramanan R, Starzl T, Ptachcinski RJ, Gartner JC, Rosenthal T (1984) Cyclosporine clearance in children following organ transplantation. J Clin Pharmacol 24: 412
Burckart G, Starzl T, Williams L, Sanghvi A, Gartner C, Venkataramanan R, Zitelli B, Malatack J, Urbach A, Diven W, Ptachcinski RJ, Shaw B, Iwatsuki S (1985) Cyclosporine monitoring and pharmacokinetics in pediatric liver transplant patients. Transplant Proc 17: 1172–1175
Feutren G, Papoz L, Assan R, Vialettes B, Karsenty G, Vexiau P, Du Rostu H, Rodier M, Sirmai J, Lallemand A, Bach JF (1986) Cyclosporin increases the rate and lenght of remissions in insulin-dependent diabetes of recent onset. Lancet II: 119–124
Gibaldi M (1977) Biopharmaceutics and clinical pharmacokinetics second ed. Lea and Febiger, Philadelphia
Kahan BD, Kramer WG, Williams C, Wideman CA (1986) Application of Bayesian forecasting to predict appropriate cyclosporine dosing regimens for renal allograft recipients. Transplant Proc 18: 200–203
Keown PA, Stiller CR, Laupacis AL, Howson W, Coles R, Stawecki M, Koegler J, Carruthers G, McKenzie N, Sinclair NR (1982) The effects and side effects of cyclosporine: relationship to drug pharmacokinetics. Transplant Proc 14: 659–661
Klare B, Walter JV, Hahn H, Emmrich P, Land W (1984) Cyclosporin in renal transplantation in children. Lancet II: 692
Lemaire M, Maurer G, Wood AJ (1986) Pharmacokinetics and metabolism. Prog Allergy 38: 93–107
Lensmeyer GL, Fields BL (1985) Improved liquid-chromatographic determination of cyclosporine with concomitant detection of a cell bound metabolite. Clin Chem 31: 196–201
Lithell H, Odlind B, Selinus A, Lindberg B, Lindström B, Frödin (1986) Is the plasma lipoprotein pattern of importance for treatment with cyclosporine? Transplant Proc 18: 50–51
Maiorca R, Cristinelli L, Scolari F, Sandrini S, Savoldi S, Brunori G, Prati E, Lojacono L, Salerni B, Tonini G (1985) Cyclosporine toxicity can be minimized by careful monitoring of blood levels. Transplant Proc 17: 4–59
Marshall WA, and Tanner JM (1966) Variations in the pattern of pubertal changes in boys. Arch Dis Child 44, 291–303
Marshall WA, and Tanner JM (1970) Variations in the pattern of pubertal changes in boys. Arch Dis Child 45: 13–23
Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakala TR (1985) The effect of food on cyclosporine absorption. Transplantation 40: 174–176
Ptachcinski RJ, Burckart GJ, Rosenthal JT, Venkataramanan R, Howrie DL, Taylor RJ, Avner ED, Ellis D, Hakala TR (1986a) Cyclosporine pharmacokinetics in children following cadaveric renal transplantation. Transplant Proc 18: 766–767
Ptachcinski RJ, Venkataramanan R, Burckart GJ (1986b) Clinical pharmacokinetics of cyclosporine. Clin Pharmacokinet 11: 107–132
Stiller CR, Dupré J, Gent M, Jenner MR, Keown PA, Laupacis A, Martell R, Rodger NW, Graffenried BV, Wolfe BMJ (1984) Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science 223: 1362–1367
The lipid research clinics program epidemiology committee (1979) Plasma lipid distributions in selected north american populations: the lipid research clinics program prevalence study. Circulation 60: 427–439
Yee GJ, Lennon TP, Gmur DJ, Kennedy MS, Deeg HJ (1986) Age dependent cyclosporine pharmacokinetics in marrow transplant recipients. Clin Pharmacol Ther 40: 438–443
Yee GJ, Lennon TP, Gmur DJ, Kennedy MS, Deeg HJ (1987) Effect of age on cyclosporine pharmacokinetics in marrow transplant recipients. Transplant Proc 19: 1704–1705
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Misteli, C., Rey, E., Pons, G. et al. Pharmacokinetics of oral cyclosporin A in diabetic children and adolescents. Eur J Clin Pharmacol 38, 181–184 (1990). https://doi.org/10.1007/BF00265981
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00265981