Summary
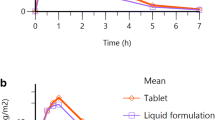
Plasma levels and the area under the plasma concentration-time curve (AUC) values of 6-mercaptopurine (6-MP) were determined in a balanced crossover study of oral (powder) and rectal (macrogol suppository) administration to 5 children with acute lymphoblastic leukaemia (ALL). The AUC (538.6 ng · h · ml−1) after the rectal dose of 30 mg/m2 was approximately 1.5-times of that (365.5 ng · h · ml−1) after the oral dose of 87.5 mg/m2. The coefficients of variation of interindividual variability of the AUCs were 21.5% and 32.3%, respectively. The relative bioavailability of the macrogol suppository compared to the powder was approximately 4.39. These findings indicate that rectal administration of 6-MP could avoid the first-pass effect of this drug in the alimentary canal and/or liver, resulting in a large AUC of 6-MP, and so could reduce interindividual variability in plasma 6-MP concentrations. Rectal administration of 6-MP may be more effective than empirical oral dosing for the treatment of children with ALL, especially for patients with nausea and/or vomiting.
Similar content being viewed by others
References
Calabresi P, Parks RE Jr (1985) Antiproliferative agents and drugs used for immunosuppression; Purine analogs. In: Gilman AG, Goodman LS, Rall TW, Murad F (eds) The pharmacological basis of therapeutics, 7th edn Macmillan, New York, pp 1275–1276
Children's Cancer and Leukemia Study Group (1988) Comparison of intermittent or continuous methotrexate plus 6-mercaptopurine in regimens for standard-risk acute lymphoblastic leukemia in childhood (JCCLSG-S811). Cancer 61: 1292–1300
Collins AP, Hohman JR, Zopf LC (1957) Polyethylene glycols as suppository bases. Am Prof Pharm 23: 231–234, 282
Endresen L, Lie SO, Storm-Mathisen I, Rugstad HE, Stokke O (1990) Pharmacokinetics of oral 6-mercaptopurine: Relationship between plasma levels and urine excretion of parent drug. Ther Drug Monit 12: 227–234
Hayder S, Lafolie P, Björk O, Peterson C (1989) 6-Mercaptopurine plasma levels in children with acute lymphoblastic leukemia: relation to relapse risk and myelotoxicity. Ther Drug Monit 11: 617–622
Kato Y, Matsushita T, Yokoyama T, Mohri K (1991a) Determination of 6-mercaptopurine in acute lymphoblastic leukemia patients' plasma by high performance liquid chromatography. Ther Drug Monit 13: 220–225
Kato Y, Matsushita T, Chiba K, Hijiya N, Yokoyama T, Ishizaki T (1991b) Dose-dependent kinetics of oral 6-mercaptopurine in children with leukemia. J Pediatr 119: 311–316
Koren G, Langevin AM, Olivieri N, Giesbrecht E, Zipursky A, Greenberg M (1990a) Diurnal variation in the pharmacokinetics and myelotoxicity of mercaptopurine in children with acute lymphocytic leukemia. Am J Dis Child 144: 1135–1137
Koren G, Ferrazini G, Sulh H, Langevin AM, Kapelushnik JK, Klein J, Giesbrecht E, Soldin S, Greenberg M (1990b) Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med 323: 17–21
Lafolie P, Hayder S, Björk O, Åhström L, Liliemark J, Peterson C (1986) Large interindividual variations in the pharmacokinetics of oral 6-mercaptopurine in maintenance therapy of children with acute leukemia and non-Hodgkin lymphoma. Acta Paediatr Scand 75: 797–803
Lafolie P, Björk O, Hayder S, Åhström L, Peterson C (1989) Variability of 6-mercaptopurine pharmacokinetics during oral maintenance therapy of children with acute leukemia. Med Oncol Tumor Pharmacother 6: 259–265
Lönnerholm G, Kreuger A, Lindström B, Myrdal U (1989) Oral mercaptopurine in childhood leukemia: Influence of food intake on bioavailability. Pediatr Haematol Oncol 6: 105–112
Miller DR, Leibin S, Alvo V (1980) The use of prognostic factors in improving the design and efficiency of clinical trials in childhood leukemia. Cancer Chemother Rep 64: 381–392
Poplack DG (1985) Acute lymphoblastic leukemia in childhood. Pediatr Clin North Am 32: 669–697
Riccardi R, Balis FM, Ferrara P, Lasorella A, Poplack DG, Mastrangelo R (1986) Influence of food intake on bioavailability of oral 6-mercaptopurine in children with acute lymphoblastic leukemia. Pediatr Haematol Oncol 3: 319–324
Robinson L, Sather H, Coccia P (1980) Assessment of the interrelationship of prognostic factors in childhood acute lymphoblastic leukemia. Am J Pediatr Haematol Oncol 2: 5–13
Simone JV (1980) The treatment of acute lymphoblastic leukemia. Br J Haematol 45: 1–4
Sulh H, Koren G, Whalen C, Soldin S, Zipursky A, Greenberg M (1986) Pharmacokinetic determinations of 6-mercaptopurine myelotoxicity and therapeutic failure in children with acute lymphoblastic leukemia. Clin Pharmacol Ther 40: 604–609
Van Scoik KG, Johnson CA, Porter WR (1985) The pharmacology and metabolism of the thiopurine drugs 6-mercaptopurine and azathiopurine. Drug Metabol Rev 16: 157–174
Yamaoka K, Nakagawa T, Uno T (1983) Moment analysis for disposition kinetics of several cephalosporin antibiotics in rats. J Pharm Pharmacol 35: 19–22
Zimm S, Collins JM, Riccardi R, O'Neil D, Narang PK, Chabner BA, Poplack DG (1983a) Variable bioavailability of oral mercaptopurine. Is maintenance chemotherapy in acute lymphoblastic leukemia being optimally delivered? N Engl J Med 308: 1005–1009
Zimm S, Collins JM, Riccardi R, O'Neil D, Chabner BA, Poplack DG (1983b) Inhibition of first-pass metabolism in cancer chemotherapy: Interaction of 6-mercaptopurine and allopurinol. Clin Pharmacol Ther 34: 810–817
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kato, Y., Matsushita, T., Uchida, H. et al. Rectal bioavailability of 6-mercaptopurine in children with acute lymphoblastic leukaemia: partial avoidance of “first-pass” metabolism. Eur J Clin Pharmacol 42, 619–622 (1992). https://doi.org/10.1007/BF00265925
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00265925