Summary
DON (6-diazo-5-oxo-l-norleucine), a glutamine antagonist, has been subjected to limited clinical trials since 1957. Use of drug in adults has been curtailed due to sparse reports of effectiveness as well as its dose-limiting toxicities, i.e., severe nausea, vomiting and mucositis. In earlier studies, children given DON orally in combination with 6-mercaptopurine had significant prolongation of remission of acute leukemias during maintenance therapy. As DON is acid-labile and relatively unstable in solution, oral administration does not appear to be ideal for DON. In the trial described in this report, i.v. DON therapy was studied, using i.v. chloropromazine to control vomiting, in 20 children, 17 of whom were evaluable following treatment at DOn dose levels ranging from 150 mg/m2 to 520 mg/m2. Nausea and vomiting, the doselimiting toxicity for adults, was controlled with chlorpromazine. Mucositis, which has also been observed in adults, did not occur in the children given DON i.v. A maximum tolerated dose was not defined; however, the projected maximum tolerated dose appears to be in excess of 450 mg/m2.
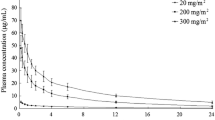
DON was measured in plasma using a rapid-sampling HPLC procedure. The total body clearance, plasma t1/2, and area under the plasma concentration curve (AUC) were calculated using a noncompartmental method. The drug is rapidly cleared from plasma (t1/2=3 h), and its volume of distribution is approximately twice that of total body water in children. These pharmacokinetic data, differ from that of adults reported by others. Specifically, the plasma t1/2 for children is longer: total body clearance (CI), and volume of distribution at steady state (Vss) are greater. In addition, no dose dependency of t1/2, Cl or Vss was observed in this study, and the DON pharmacokinetics were linear and predictable. Five of nine children with acute leukemia showed improvement, though insufficient for classification as partial response, and five of eight children with solid tumors also showed improvement. Further trials using DON in combination with thiopurines or other agents appear indicated.
Similar content being viewed by others
References
Barclay RK, Phillips MA (1966) Effects of 6-Diazo-5-oxo-lnorleucine and other tumor inhibitors on the biosynthesis of nicotinamide adenine dinucleotide in mice. Cancer Res 26: 282–286
Bennett LL Jr (1975) Glutamine antagonists. Hdb Exp Pharmacol 38(2): 484–522
Catane R, Von Hoff DD, Glaubiger DL (1979) Azaserine, DON, and azotomycin: three diazo analogs of l-glutamine with clinical antitumor activity. Cancer Treat Rep 63: 1033–1038
Dion HW, Fusari SA, Jakubowski ZL (1965) 6-Diazo-5-oxo-lnorleucine, a new tumor-inhibitory substance: 11. Isolation and characterization. J Am Chem Soc 78: 3075–3077, 1965
Ehrlich J, Coffey GL, Fisher MW (1956) 6-Diazo-5-oxo-l-norleucine, a new tumor-inhibitory substance. 1. Biologic studies. Antibiot Chemother 6: 487–497
Eidinoff ML, Knoll JE, Marano B (1958) Pyrimidine studies: 1. Effect of DON (6-Diazo-5-oxo-1-norleucine) on incorporation of precursor into nucleic acid pyrimidines. Cancer Res 18: 105–109
Ellis DB, Sommar KM (1972) Biosynthesis of respiratory tract mucins: 11. Control of hexosamine metabolism by 1-glutamine: d-fructose 6-phosphate aminotransferase. Biochim Biophys Acta 276: 105–112
Hewlett JS, Battle JD Jr, Bishop RC (1964) Phase II study of A-8103 (NSC-25154) in acute leukemia in adults. Cancer Chemother Rep 42: 25–28
Jackson D, Robson JM, Wander ACE (1959) The effect of 6-Diazo-5-oxo-l-norleucine (DON) on pregnancy. J Endocrinol 18: 204–207
Jayaram HN, Cooney DA, Milman HA (1976) DON, CONV and DONV: I. Inhibition of 1-asparaginase synthetase in vitro. Biochem Pharmacol 25: 1571–1582
Kovach JS, Eagen RT, Powis G (1981) Phase I and pharmacokinetic studies of DON. Cancer Treat Rep 65: 1031–1036
Levenberg B, Melnick I, Buchanan JM (1957) Biosynthesis of the purines. XV. The effect of aza-1-serine and 6-Diazo-5-oxo-1-norleucine on inosinic acid biosynthesis de novo. J Biol Chem 225: 163–176
Li MC (1961) Management of choriocarcinoma and related tumors of uterus and testis. Med Clin North Am 45: 661–676
Livingston RB, Venditti JM, Cooney DA (1970) Glutamine antagonists in cancer chemotherapy. In: Garattini S, Goldin A, Hawking F, Kopin IJ (eds) Advances in pharmacology and chemotherapy. Academic Press, New York, pp 57–120
Magill GB, Myers WPL, Reilly MC (1957) Pharmacological and initial therapeutic observations on 6-Diazo-5-oxo-1-norleucine (DON) in human neoplastic disease. Cancer 10: 1138–1150
National Cancer Institute (1979) Clinical brochure. DON. NSC 7365. Investigative Drug Branch, Cancer Therapy Evaluation Program, DCT, NCI, Bethesda, Md
Nelson JA, Drake S (1974) Potentiation of methotrexate toxicity by dipyridamole. Cancer Res 44: 2492–2496
Nelson JA, Herbert B (1981) Rapid analysis of 6-Diazo-5-oxo-1-norleucine (DON) in human plasma and urine. J Liquid Chromatogr 4: 1641–1649
Nelson JA, Parks RE Jr (1972) Biochemical mechanisms for the synergism between 6-thioguanine and 6-(methylmercapto) purine ribonucleoside in sarcoma 180 cells. Cancer Res 32: 2034–2041
Paterson ARP, Wang MC (1970) Mechanism of the growth inhibition potentiation arising from combination of 6-mercaptopurine with 6-(methylmercapto) purine ribonucleoside. Cancer Res 30: 2379–2387
Potter M (1958) Variation in resistance patterns in different neoplasms. Ann NY Acad Sci 76: 630–642
Rocci ML, Vusko WJ (1983) LAGRAN program for area and moments in pharmacokinetic analysis. Comput Prog Biomed 16: 203–216
Rosenbluth RJ, Cooney DA, Jayaram HN (1976) Inhibition of 1-asparaginase synthetase in vivo. Biochem Pharmacol 25: 1851–1858
Sartorelli AC (1965) Approaches to the combination chemotherapy of transplantable neoplasms. Prog Exp Tumor Res 6: 228–288
Sklaroff RB, Casper ES, Magill GB (1981) Phase I Study of 6-Diazo-5-oxo-1-norleucine (don). Cancer Treat Rep 64: 1247–1251
Sullivan MP, Beatty EC Jr, Hyman CB (1962) A comparison of the effectiveness of standard dose 6-mercaptopurine, combination 6-mercaptopurine and DON, and high-loading 6-mercaptopurine therapies in treatment of the acute leukemias of childhood: results of a cooperative study. Cancer Chemother Rep 18: 83–85
Veterans Administration Cancer Chemotherapy Study Group (1959) A elinical study of the comparative effect of nitrogen mustard and DON in patients with bronchogenic sarcoma, Hodgkin's disease, lymphosarcoma, and melanoma. J Nat Cancer Inst 22: 433–439
Author information
Authors and Affiliations
Additional information
Supported by Contract NO1-CM-07342-22 from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Rights and permissions
About this article
Cite this article
Sullivan, M.P., Nelson, J.A., Feldman, S. et al. Pharmacokinetic and phase I study of intravenous DON (6-diazo-5-oxo-L-norleucine) in children. Cancer Chemother. Pharmacol. 21, 78–84 (1988). https://doi.org/10.1007/BF00262746
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00262746