Summary
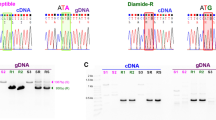
We report the cloning and sequencing of a glutathione S-transferase (GST) gene from the housefly Musca domestica. A cDNA λgt11 library was prepared from the organophosphate insecticide-resistant housefly strain Cornell-R — a variant that has elevated GST activity. The λ phage GST clone was identified on the basis of its ability to cross-hybridize to a GST DNA probe from Drosophila melanogaster. Based on amino acid homology to other GSTs and expression of GST activity in Escherichia coli, the Musca GST gene (MdGST-1) belongs to the GST gene family. Although organophosphate resistance in Cornell-R is largely due to one of the GSTs, MdGST-1 is probably not the enzyme responsible for resistance. The mutation that controls resistance to organophosphate insecticides in Cornell-R is highly unstable and we isolated spontaneous variants to both insecticide sensitivity and to even higher levels of resistance. This provided us with an isogenic set of three strains. We found that MdGST-1 transcript levels as measured by Northern assays are higher in all three Cornell-R strains relative to the sensitive wild type, but that the sensitive Cornell-R strain has more MdGST-1 transcript than does the highly resistant Cornell-R strain. These data as well as Southern analysis of genomic DNA allow us to conclude: (1) there are multiple GST genes in M. domestica; (2) the natural variant Cornell-R excess transcript from two and probably more of these genes; and (3) the unstable mutation in Cornell-R influences the levels of multiple GSTs.
Similar content being viewed by others
References
Askelof P, Guthenberg C, Jakobson I, Mannervick B (1975) Purification and characterization of two glutathione S-aryltransferase activities from rat liver. Biochem J 147:513–522
Board PG, Webb GC (1987) Isolation of a cDNA clone and localization of human glutathione S-transferase 2 genes to chromosome band 6 p 12. Proc Natl Acad Sci USA 84:2377–2381
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Clark AG, Dauterman WC (1982) Characterization by affinity chromatography of glutathione S-transferases from different strains of housefly. Pest Biochem Physiol 17:307–314
Clark AG, Shamaan NA (1984) Evidence that DDT-dehydrochlorinase from the housefly is a glutathione S-transferase. Pest Biochem Physiol 22:249–261
Clark AG, Smith JN, Speir TW (1973) Cross specificity of some vertebrate and insect glutathione S-transferases with methyl parathion, CDNB and S-crotonyl-N-acetylcysteamine as substrates. Biochem J 135:385–392
Clark AG, Cropp P, Smith J, Speir T, Tan B (1976) Photometric determination of methyl parathion GSH S-methyl transferase. Pest Biochem Physiol 6:126–131
Clark AG, Shamaan NA, Dauterman WC, Hayaoka T (1984) Characterization of multiple glutathione S-transferases from the housefly, Musca domestica. Pest Biochem Physiol 22:51–59
Cochrane BJ, Morrissey JJ, LeBlanc GA (1987) The genetics of xenobiotic metabolism in Drosophila-IV. Insect Biochem 17:731–738
Grove G, Zarlengo RP, Timmerman KP, Li NQ, Tam ME, Tu CPD (1988) Characterization and heterospecific expression of cDNA clones of genes in the maize GSH S-transferase multigene family. Nucleic Acid Research 16:425–438
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Iverson PL, Mata JE, Hines N (1987) Rapid isolation of both RNA and DNA from cultured cells or whole tissues with a benchtop ultracentrifuge. Biotechniques 5:521–524
Kano T, Sakai M, Muramatsu M (1987) Structure and expression of a human class π glutathione S-transferase messenger RNA. Cancer Res 47:5626–5630
LaRoche S, Leisinger T (1990) Sequence analysis and expression of the bacterial dichloromethane dehalogenase structural gene, a member of the glutathione S-transferase supergene family. J Bacteriol 172:164–171
MacDonald RJ, Swift GH, Przybyla AE, Chirgwin JM (1987) Isolation of RNA using guanidinium salts. Methods Enzymol 152:224–226
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Motoyama N, Dauterman W (1975) Interstrain comparison of glutathione-dependent reactions in susceptible and resistant houseflies. Pest Biochem Physiol 5:489–495
Motoyama N, Dauterman W (1977) Purification and properties of housefly glutathione S-transferase. Insect Biochem 7:361–369
Motoyama N, Dauterman W (1978) Mol weight, subunits and multiple forms of GSTase from the housefly. Insect Biochem 8:337–348
Motoyama N, Dauterman W, Plapp FW Jr (1977) Genetic studies on glutathione-dependent reactions in resistant strains of the housefly. Pest Biochem Physiol 7:443–450
Mouches C, Pasteur N, Berge J, Hyrien O, Raymond M, Saint Vincent B, Silverstri M, Georghiou G (1986) Amplification of an esterase gene is responsible for insecticide resistance in the California Culex mosquito. Science 233:778–780
Oppenoorth F, Rupes V, El Bashir S, Houx N, Voerman S (1972) Glutathione-dependent degradation of parathione and its significance for resistance in the housefly. Pest Biochem Physiol 2:262–277
Oppenoorth F, Smissaert H, Welling W, van der Pas L, Hitman K (1977) Insensitive acetylcholinesterase, high glutathione Stransferase and hydrolytic activity as resistance factors in a TCVP-resistant strain of housefly. Pest Biochem Physiol 7:3447
Ottea JA, Plapp FW (1984) Gluthathione S-transferase in the house fly: biochemical and genetic changes associated with induction and insecticide resistance. Pest Biochem Physiol 22:203–208
Pickett CB, Lu AYH (1989) Glutathione S-transferases: Gene structure, regulation and biological function. Annu Rev Biochem 58:743–764
Plapp FW (1984) Genetic basis of insecticide resistance in the housefly. Pest Biochem Physiol 22:194–201
Smith DB, Davem KM, Board PG, Tin WU, Garcia BG, Mitchell GR (1986) MR 26,000 antigen of Schistosoma japonicum recognized by resistant WEHI 129/J mice is a parasite glutathione S-transferase. Proc Natl Acad Sci USA 83:8703–8707
Tripathi R, O'Brien R (1973) Insensitivity of acetylcholinesterase as a factor in resistance in the housefly. Pest Biochem Physiol 2:262–277
Tuong Y-PS, Hsieh T-S, Tu C-PD (1990) Drosophila glutathione S-transferase 1-1 shares a region of sequence homology with the maize glutathione S-transferase III Proc Natl Acad Sci USA 87:31–35
Author information
Authors and Affiliations
Additional information
Communicated by D.J. Finnegan
Rights and permissions
About this article
Cite this article
Wang, Jy., McCommas, S. & Syvanen, M. Molecular cloning of a glutathione S-transferase overproduced in an insecticide-resistant strain of the housefly (Musca domestica). Molec. Gen. Genet. 227, 260–266 (1991). https://doi.org/10.1007/BF00259679
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00259679