Summary
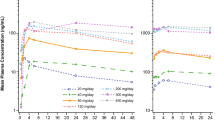
The pharmacokinetics of the new antifolate CB 3717 were studied in 20 patients during its phase-I clinical evaluation. The drug was administered at doses of 100–550 mg/m2 in 1-h and 12-h infusions, resulting in peak plasma concentrations of CB 3717 of 40–200 μM. There was a linear relationship between the dose and both CB 3717 AUC and peak plasma levels. Following a 1-h infusion, drug levels in the plasma decayed biphasically (t1/2α=49±9 min, t1/2β=739±209 min). 27%±2% of the dose was excreted in urine in the 24-h period after treatment, suggesting that the major route of elimination was via the bile. Furthermore, the parent compound CB 3717 and its desglutamyl metabolite, CB 3751, were found in a faecal collection although the metabolite was not detected in plasma or urine samples. Plasma protein binding of CB 3717 was extensive (97.6%±0.1%). Significant quantities of CB 3717 penetrated into ascitic fluid but not into cerebrospinal fluid.
Residual drug was detected in postmortem kidney tissue from a patient who died of progressive disease 8 days after treatment with 330 mg/m2 CB 3717. Thus, dose-limiting renal toxicity (maximum tolerated dose 600 mg/m2) may be due to drug precipitation in the renal tubules. Elevation of liver enzymes, in particular transaminases, occurred frequently as a toxic manifestation of CB 3717 therapy. In 11 patients studied after their first treatment there was a positive correlation between the rise in serum alanine transaminase and peak drug levels (r=0.69, P=0.02)
These pharmacokinetic studies have shown that, by analogy with experimental systems, cytotoxic plasma levels of CB 3717 are archieved in man. In addition, they have been valuable in interpreting toxicities observed during phase-I clinical studies.
Similar content being viewed by others
References
Alison DL, Robinson B, Harland SJ, Evans BD, Calvert AH (1984) Phase I clinical trial of CB 3717 (N-(4-(N((2-amino-4-hydroxy-6-quinazolinyl)methyl)prop-2-ynylamino)benzoyl)-l-glutamic acid). Br J Cancer 50:242
Breithaupt H, Kuenzlen E (1982) Pharmacokinetics of Methotrexate and 7-hydroxymethotrexate following infusion of high-dose methotrexate. Cancer Treat Rep 66:1733–1741
Calvert AH, Bondy PK, Harrap KR (1977) Some observations on the human pharmacology of methotrexate. Cancer Treat Rep 61:1647–56
Calvert AH, Alison DL, Harland SJ, Jackman AL, Mooney CJ, Smith IE, Harrap KR (1983) Phase I studies with CB 3717 (N-(4-(N((N-amino-4-hydroxy-6-quinazolinyl)methyl)prop-2-ynylamino)benzoyl)-l-glutamic acid). Br J Cancer 48:116
Christophidis N, Louis WJ, Lucas I, et al. (1981) Renal clearance of methotrexate in man during high dose oral and IV infusion therapy. Cancer Chemother Pharmacol 6:59–64
Diddens H, Niethammer D, Jackson RC (1983) Patterns of cross resistance to the antifolate drugs trimetrexate, metoprine, homofolate and CB 3717 in human lymphoma and osteosarcoma cells resistent to methotrexate. Cancer Res 43:5286–5292
Isacoff WH, Morrison PF, Aroesty J, Willis KL, Block JB, Lincoln TL (1977) Pharmacokinetics of high dose methotrexate with citrovorum factor rescue. Cancer Treat Rep 61:1665–1674
Jackman AL, Calvert AH, Hart LI, Harrap KR (1983) Inhibition of thymidylate synthetase by the new quinazoline antifolate CB3717; enzyme preparation and kinetics. In: Purine metabolism in man, vol IV, part B: Biochemical immunological and cancer research. Plenum, New York, pp 375–378
Jackson RC, Jackman AL, Calvert AH (1983) Biochemical effects of a quinazoline inhibitor of thymidylate synthetase N-(4-(N((2-amino-4-hydroxy-6-quinazolinyl)methyl)prop-2-ynylamino)benzoyl)-l-glutamic acid (CB 3717) on human lymphoblastoid cells. Biochem Pharmacol 32:3783–3790
Jacobs SA, Stoller RG, Chabner BA, Johns DC (1976) 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J Clin Invest 57:534–538
Jaffe N, Traggis D (1975) Toxicity of high dose methotrexate and citrovorum factor in osteogenic sarcoma. Cancer Chemother Rep [3] 6:31–36
Jones TR, Calvert AH, Jackman AL, Brown SJ, Jones M, Harrap KR (1981) A potent antitumour quinazoline inhibitor of thymidylate synthetase: synthesis, biological properties and therapeutic results in mice. Eur J Cancer 15:11–19
Loo JCK, Riegelman S (1970) Assessment of pharmacokinetic constants from post infusion blood curves obtained after iv infusion. J Pharm Sci 59:53–55
Newell DR, Siddik ZH, Calvert AH, Jackman AL, Alison DL, McGhee KG, Harrap KR (1982) Pharmacokinetics and toxicity studies with CB 3717. Proc Am Assos Cancer Res 23:181
Newell DR, Siddik ZH, McGhee KG, Jackman AL, Calvert AH, Harrap KR (1982) Pharmacokinetic and toxicity studies with CB3717. Br J Cancer 46:467
Nirenberg A, Mosende C, Mehta B, Gisolfi AL, Rosen G (1977) High dose methotrexate with citrovorum rescue: predictive value of serum methotrexate concentration and corrective measures to avert toxicity. Cancer Treat Rep 61:779
Ottaway JH (1973) Normalization in the fitting of data by iterative methods. Biochem J 134:729
Pratt CB, Roberts D, Shanks E, Warmath EL (1975) Response, toxicity and pharmacokinetics of high dose methotrexate with citrovorum factor rescue for chidren with osteosarcoma and other malignant tumours. Cancer Chemother Rep 1:13–18
Rosen G, Suwansirikul S, Kwon C, Tan C, Wu SJ, Beattie EJ, Murphy ML (1974) High dose methotrexate with citrovorum factor rescue and adriamycin in childhood osteogenic sarcoma. Cancer 33:1151–1163
Sampson J (1970) Non linear squares program BMDX85, In: BMD Biomedical Computer Programs X-Series Supplement. ed WJ Dixon, University of California Press, Berkeley, California, pp 177–186
Storer AC, Darlison MG, Cornish-Bowden A (1975) The nature of experimental error in enzyme kinetic measurements. Biochem J 151:361–367
Wagner JC (1975) Fundamentals of clinical pharmacokinetics. Drug Intelligence publications, Hamilton p 82
Author information
Authors and Affiliations
Additional information
This work was supported by grants from the Medical Research Council and Cancer Research Campaign, U. K.
Rights and permissions
About this article
Cite this article
Alison, D.L., Newell, D.R., Sessa, C. et al. The clinical pharmacokinetics of the novel antifolate N10-propargyl-5,8-dideazafolic acid (CB 3717). Cancer Chemother. Pharmacol. 14, 265–271 (1985). https://doi.org/10.1007/BF00258131
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00258131