Abstract
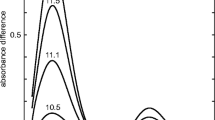
Raman and infrared spectroscopy have been simultaneously applied, for the first time, to the study of myelin membranes and their proteolipid protein (PLP) so as to obtain information on the secondary structure of proteins and the ordering of lipid chains. The vibrational spectra were recorded at physiological pH using a non-denaturing detergent (n-octyl-β-d-glucopyranoside) in phosphate buffer. Neither the buffer nor the detergent interfere spectroscopically with the amide bands from proteins. The spectra reveal that the predominant secondary structure in the polypeptide backbone in myelin is the helix. The proteolipid protein was found to be more disordered than the polypeptide arrangement of the myelin membrane, as deduced from the relative intensities and halfwidths of characteristic infrared amide I bands. β-form and turns are also present, the amount of these structures being higher in PLP. The study of the Raman spectra of vC-C and vC-H regions made it possible to obtain information on the lipid chain order.
Similar content being viewed by others
References
Aguilar JS, Cózar M, Criado M, Monreal J (1982) Method for lyophilizing brain proteolipid preparations that increases subsequent solubilization by detergents. J Neurochem 39:1733–1736
Boggs JM, Moscarello MA (1978) Effect of basic protein from human central nervous system myelin on lipid bilayer structure. J Membr Biol 39:75–96
Bullock JO, Cohen FS (1986) Octyl-glucoside promotes incorporation of channels into neutral planar phospholipid bilayers. Studies with colicin Ia. Biochim Biophys Acta 856:101–108
Byler DM, Susi H (1986) Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 25:469–487
Cockle SA, Epand RM, Boggs JM, Moscarello MA (1978) Circular dichroism studies on lipid-protein complexes of a hydrophobic myelin protein. Biochemistry 17:624–629
Cortijo M, Alonso A, Gómez-Fernández JC, Chapman D (1982) Intrinsic protein-lipid interactions. Infrared spectroscopic studies of gramicidin A, bacteriorhodopsin and Ca2+-ATPase in biomembranes and reconstituted systems. J Mol Biol 157:597–618
Curatolo W, Verma SP, Sakura JD, Small DM, Shipley GG, Wallach DFH (1978) Structural effects of myelin proteolipid apoprotein on phospholipids: a Raman spectroscopic study. Biochemistry 17:1802–1807
De Lozé C, Baron MH, Fillaux F (1978) Interactions of the CONH group in solution. Interpretation of the infrared and Raman spectra in relationship to secondary structures of peptides and proteins. J Chem Phys 75:631–649
Folch-Pi J, Lees M (1951) Proteolipids, a new type of tissue lipoproteïns. Their isolation from brain. J Biol Chem 191:807–817
Frushour BG, Koenig JL (1975) Raman spectroscopy of proteins. In: Clark, RJH, Hester RE (eds) Advances in infrared and Raman spectroscopy. John Wiley and Sons, New York, pp 35–97
García-Segura LM, de Cózar M, Moreno MC, Monreal J (1986) Freeze-fracture characterization of proteolipid protein and basic protein of central nervous system myelin incorporated to liposomes. Brain Res 380:261–266
Hellenius A, Fries E, Kartenbeck (1977) Reconstitution of Semliki fores virus membrane. J Cell Biol 75:866–880
Hellenius A, McCaslin DR, Fries E, Tanford CH (1979) Properties of detergents. In: Fleischer S, Packer L (eds) Methods in enzymology, vol 56. Academic Press, New York, pp 734–749
Jenkinson TJ, Kamat VB, Chapman D (1969) Physical studies of myelin II. Proton magnetic resonance and infrared spectroscopy. Biochim Biophys Acta 183:427–433
Kirschner D, Ganser AL, Caspar DLD (1984) Diffraction studies of molecular organization and membrane interactions in myelin. In: Morell P (ed) Myelin. Plenum Press, New York, pp 51–95
Larsson K, Rand RP (1973) Detection of changes in the environment of hydrocarbon chains by Raman spectroscopy and its application to lipid-protein systems. Biochim Biophys Acta 326:245–255
Lavialle F, Levin IW (1980) Raman spectroscopic study of the interactions of dimyristoyl-and 1-palmitoyl-2-oleoyl-phosphatidylcholine liposomes with myelin proteolipid apoprotein. Biochemistry 19:6044–6050
Levin IW (1984) Vibrational spectroscopy of membrane assemblies. In: Clark RJH, Hester RE (eds) Advances in infrared and Raman spectroscopy. John Wiley and Sons, New York, pp 1–48
Levin IW, Keihn E, Harris WC (1985) A Raman spectroscopic study on the effect of cholesterol on lipid packing in diether phosphatidylcholine bilayer dispersions. Biochim Biophys Acta 820:40–47
Lippert JL, Gorczyca LE, Meiklejohn G (1975) A laser Raman spectroscopic investigation of phospholipid and protein configurations in hemoglobin-free erythrocyte ghosts. Biochim Biophys Acta 382:51–57
MacPhail RA, Strauss HL, Snyder RG, Elliger CA (1984) C-H stretching modes and the structure of n-alkyl chains. 2. Long, all-trans chains. J Phys Chem 88:334–341
Michel H (1983) Crystallization of membrane proteins. Trends Biochem Sci 8:56–59
Milanovich FP, Shore B, Harney RC (1976) Raman spectroscopic analysis of Dutch Belt rabbit erythrocyte ghosts. Chem Phys Lipids 17:79–84
Monreal J (1975) Chromatographic fraction of brain white matter proteolipid. J Neurochem 25:539–541
Morell P (1984) Myelin. Plenum Press, New York
Norton WT (1984) Oligodendroglia. Advances in neurochemistry, vol 5. Plenum Press, New York, pp 47–75
O'Brien J, Sampson EL (1965) Lipid composition of the normal human brain: gray matter, white matter and myelin. J Lipid Res 6:537–544
Parker FS (1971) Applications of infrared spectroscopy in biochemistry, biology and medicine. Hilger, London
Pink DA, Green TJ, Chapman D (1980) Raman scattering in bilayer of saturated phosphatidylcholines. Experiment and theory. Biochemistry 19:349–356
Ramos JM, Carmona P, Monreal J, Chapman D (1986) Raman and infrared spectroscopic study of myelin membranes. J Mol Struct 143:461–464
Smith R, Cook J, Dickens PA (1984) Structure of the proteolipid protein extracted from bovine central nervous system myelin with non-denaturing detergents. J Neurochem 42:306–313
Snyder RG, Scherer JR, Gaber BP (1980) Effects of chain packing and chain mobility on the Raman spectra of biomembranes. Biochim Biophys Acta 601:47–53
Stoffel W, Giersiefen H, Hillen H, Schroeder W, Tunggal B (1985) Amino acid sequence of human and bovine brain myelin proteolipid protein (lipophilin) is completely conserved. Biol Chem Hoppe-Seyler 366:627–635
Tu A (1982) Raman spectroscopy in biology. John Wiley and Sons, New York
Van Wart HE, Lewis A, Scheraga HA, Saeva FD (1973) Disulfide bond dihedral angles from Raman spectroscopy. Proc Natl Acad Sci USA 70:2619–2623
Verma SP, Wallach DFH (1977) Raman spectra of some saturated, unsaturated and deuterated C18 fatty acids in the HCH-deformation and CH-stretching regions. Biochim Biophys Acta 486:217–227
Verma SP, Wallach DFH, Sakura JD (1980) Raman analysis of the thermotropic behavior of lecithin-fatty acid systems and of their interaction with proteolipid apoprotein. Biochemistry 19:574–579
Waehneldt, TV, Mandel P (1970) Proteins of rat brain myelin. Extraction with sodium dodecylsulphate and electrophoresis on analytical and preparative scale. FEBS Lett 9:209–212
Wallach DFH, Verma SP (1975) Raman and resonance Raman scattering by erythrocyte ghosts. Biochim Biophys Acta 382:542–551
Watts A, De Pont JHM (1985) Progress in protein-lipid interactions, vol 1. Elsevier, Amsterdam, pp 1–56
Wood DD, Moscarello MA (1984) Is the myelin membrane abnormal in multiple sclerosis? J Membr Biol 79:195–201
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ayala, G., Carmona, P., de Cózar, M. et al. Vibrational spectra and structure of myelin membranes. Eur Biophys J 14, 219–225 (1987). https://doi.org/10.1007/BF00256355
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00256355