Summary
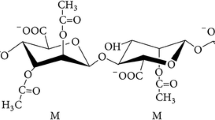
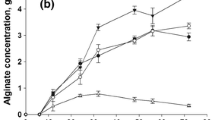
In this study, some factors influencing the secretion of alginic acid and the accumulation of poly-β-hydroxybutyrate (PHB) by a highly mucoid strain of Azotobacter vinelandii were investigated in batch culture. The highest alginate yields (6.0–7.5 mgml-1 culture supernate) were routinely obtained for growth in a phosphate and nitrogen-rich medium (PNR) with glucose as carbon source, aerated by shaking at 280 rpm. In this case, the intracellular accumulation of PHB reached a maximum of 30% cell dry weight. At 120 rpm alginate yield was only 1.4 mgml-1 and PHB constituted 40%. This reflected oxygen-limitation under these conditions. The presence of inorganic phosphate in PNR was seen to be important as growth in low-salts, nitrogen-free medium resulted in poor alginate production, which was not improved by addition of nitrogen sources, such as nitrate or glutamate. Under otherwise optimised conditions, different peptones gave widely different alginate yields. If glucose was replaced by sucrose, growth and alginate production were reduced.
Similar content being viewed by others
References
Anderson AJ, Hacking AJ, Dawes EA (1987) Alternative pathways for the biosynthesis of alginate from fructose and glucose in Pseudomonas mendocina and Azotobacter vinelandii. J Gen Microbiol 133:1045–1052
Chen WP, Chen JY, Chang SC, Su CL (1985) Bacterial alginate produced by a mutant of Azotobacter vinelandii. Appl Environ Microbiol 49:543–546
Davidson IW, Sutherland IW, Lawson CJ (1977) Localisation of O-acetyl groups of bacterial alginate. J Gen Microbiol 98:603–608
Deavin L, Larman TR, Lawson CJ, Righelato RC, Slocombe S (1977) The production of alginic acid by Azotobacter vinelandii in batch and continuous culture. In: Sandford PA, Laskin A (eds) Extracellular microbial polysaccharides. American Chemical Society, Washington D.C., pp 14–26
Drozd JW, Tubb RS, Postgate JR (1972) A chemostat study on the effect of fixed nitrogen sources on nitrogen fixation, membranes and free amino acids in Azotobacter chroococum. J. Gen Microbiol 73:221–232
Evans LR, Linker A (1973) Production and characterisation of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol 116:915–924
Gorin PAJ, Spencer JFT (1966) Exocellular alginic acid from Azotobacter vinelandii. Can J Chem 44:993–998
Hacking AJ, Taylor IWF, Jarman TR, Govan JRW (1983) Alginate biosynthesis by Pseudomonas mendocina. J Gen Microbiol 129:3473–3480
Horan NJ, Jarman TR, Dawes EA (1981) Effects of carbon source and inorganic phosphate concentration on the production of alginic acid by a mutant of Azotobacter vinelandii and on the enzymes involved in its biosynthesis. J Gen Microbiol 127:185–191
Kennedy JF, Bradshaw IJ (1984) A rapid method for the assay of alginates in solution using polyhexamethylenebiguanidium chloride. Brit Polymer J 16:95–101
Klugkist J, Haaker H (1984) Inhibition of nitrogenase activity by ammonium chloride in Azotobacter vinelandii. J Bacteriol 157:148–151
Law JH, Slepecky RA (1961) Assay of poly-β-hydroxybutyric acid. J Bacteriol 82:33–36
Lin LP, Sadoff HL (1968) Encystment and polymer production by Azotobacter vinelandii in the presence of β-hydroxybutyrate. J Bacteriol 95:2336–2343
Neijssel OM, Tempest DW (1975) The regulation of carbohydrate metabolism in Klebsiella aerogenes NCTC 418 organism growing in chemostat culture. Arch Microbiol 106:251–258
Page WJ, Sadoff HL (1975) Relationship between calcium and uronic acids in the encystment of Azotobacter vinelandii. J Bacteriol 122:145–151
Parsons AB, Dugan PR (1971) Production of extracellular polysaccharide matrix by Zoogloea ramigera. Appl Microbiol 21:657–661
Senior PJ, Dawes EA (1973) The regulation of poly-b-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J 134:225–238
Skjak-Braek G, Grasdalen H, Larsen L (1986) Monomer sequence and acetylation pattern in some bacterial alginates. Carbohydr Res 154:239–250
Stephenson MP, Jackson FA, Dawes EA (1978) Further observations on carbohydrate metabolism and its regulation in Azotobacter vinelandii. J Gen Microbiol 109:89–96
Stevenson LH, Socolofsky MD (1966) Cyst formation and poly-β-hydroxybutyrate accumulation in Azotobacter. J Bacteriol 91:304–310
Sutherland IW (1977) Microbial exopolysaccharide synthesis. In: Sandford PA, Laskin A (eds) Extracellular Microbiol Polysaccharides. American Chemical Society, Washington D.C., pp 40–57
Williamson DH, Wilkinson JF (1958) The isolation and estimation of the poly-β-hydroxybutyrate inclusions of Bacillus species. J Gen Microbiol 19:198–209
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brivonese, A.C., Sutherland, I.W. Polymer production by a mucoid stain of Azotobacter vinelandii in batch culture. Appl Microbiol Biotechnol 30, 97–102 (1989). https://doi.org/10.1007/BF00256004
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00256004