Summary
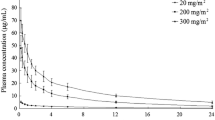
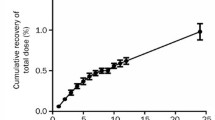
Plasma pharmacokinetics of VP16-213 were investigated after a 30–60 min infusion in 14 adult patients and six children. In adults the elimination half-life (T1/2 β), plasma clearance (Clp) and volume of distribution (Vd) were respectively 7.05±0.67 h, 26.8±2.4 ml/min/m2, and 15.7±1.8 l/m2; in children 3.37±0.5 h, 39.34±6.6 ml/min/m2, and 9.97±3.7 l/m2. After repeated daily doses no accumulation of VP16-213 was found in plasma. The unchanged drug found in the 24 h urine after administration amounted to 20–30% of the dose.
In eight choriocarcinoma patients plasma levels of VP16-213 were measured after oral capsules and drinkable ampoules. The bioavailability compared to the i.v. route was variable, mean values being 57% for capsules and 91% for ampoules. In one further patient, with abnormal d-Xylose absorption results, VP16-213 was not detectable in plasma after the oral ampoule dose.
Steady state levels investigated in three patients after 72 h continuous VP16-213 infusion (100 mg/m2/24 h) were around 2–5 μg/ml. Levels of VP16-213 were undetectable in CSF after i.v. or oral administration.
Similar content being viewed by others
References
Allen LM (1980) Analysis of 4′-Demethylepipodophyllotoxin-9-(4,6-0-ethylidene-βd-glucopyranoside) by high-pressure liquid chromatography. J Pharm Sci 69:1440
Allen LM, Creaven PJ (1975) Comparison of the human pharmacokinetics of VM26 and VP16, two antineoplastic epipodophyllotoxin glucopyranoside derivatives. Eur J Cancer 11:697
Arnold AM (1979) Podophyllotoxin derivative VP16-213. Cancer Chemother Pharmacol 3:71
Brunner KW, Sonntag RW, Ryssel HJ, Cavalli F (1976) Comparison of the biologic activity of VP16-213 given iv and orally in capsules or drink ampules. Cancer Treat Rep 60:1377
Cavalli F, Sonntag RW, Jungi F, Senn HJ, Brunner KW (1978) VP16-213 monotherapy for remission induction of small cell lung cancer: A randomized trial using three dosage schedules. Cancer Treat Rep 62:473
Colombo T, Broggini M, Torti L, Erba E, D'Incalci M (1981) Pharmacokinetics of VP16 in Lewis lung carcinoma bearing mice. Cancer Chemother Pharmacol (in press)
Creaven PJ, Allen LM (1975) EPEG, a new antineoplastic epipodophyllotoxin. Clin Pharmacol Ther 18:221
D'Incalci M, Erba E, Vaghi M, Morasea L (1982) In vitro cytotoxicity of VP16-213 on primary tumor and metastasis of levis lung carcinoma. Eur J Cancer (in press)
Evans WE, Sinkule JA, Horvath A, Crom WR, Dow LW, Rivera G (1981) Clinical pharmacology of VM26 (NSC 122819) and VP16 (NSC 1415-0) in children with cancer. Proc Am Ass Cancer Res 22:174
Farina P, Marzillo G, D'Incalci M (1981) High-performance liquid chromatography determination of 4′-demethylepipodophyllotoxin-9-(4,6-0-ehylideneβ-d-glucopyranoside (VP16-213) in human plasma. J Chromatogr 222:141
Holthuis JJM, Pinedo HM, Van Oort WJ (1981) A sensitive high-performance liquid chromatographic method for determination of the antineoplastic agents VP16-213 and VM26 in biological fluids. Anal Chim Acta 130:23
Issell BF, Crooke ST (1979) Etoposide (VP16-213). Cancer Treat Rev 6:107
Newlands ES, Ragshawe KD (1980) Anti-tumor activity of the epipodophyllin derivative VP16-213 (etoposide: NSC 141540) in gestational choriocarcinoma. Eur J Cancer 16:401
Pinkerton CR, Glasgow JFT, Bridges JM, Welshman SG (1981) Enterotoxic effect of methotrexate: Does it influence the drug's absorption in children with acute lymphoblastic leukaemia? Br Med J 282:1276
Rivera G, Avery T, Pratt C (1975) 4′-Demethylepipodophyllotoxin 9-(4,6-0-2-thenylidene-β-d-glucopyranoside) (NSC-122819; VM26) and 4′-demethylepipodophyllotoxin 9-(4,6-0-ethylidene-β-d-glucopyranoside) (NSC-141540; VP16-213) in childhood cancer: Preliminary observations. Cancer Chemother Rep 59:743
Rozencweig M, Von Hoff DD, Henney JE, Muggià FM (1977) VM26 and VP16-213: A comparative analysis. Cancer 40:334
Snodgrass W, Walker L, Heideman R, Odom LF, Hays T, Tubergen DG (1980) Kinetics of VP16 epipodophyllotoxin in children with cancer. Proc Am Ass Cancer Res 21:333
Stähelin H (1973) Activity of a new glycosidic lignan derivative (VP16-213) related to podophyllotoxin in experimental tumors. Eur J Cancer 9:215
Strife RJ, Jardine I, Colvin M (1980) Analysis of the anticancer drugs VP16-213 and VM26 and their metabolites by high-performance liquid chromatography. J Chromatogr 182:211
Strife RJ, Jardine I, Colvin M (1981) Analysis of the anticancer drugs etoposide (VP16-213) and teniposide (VM26) by highperformance liquid chromatography with fluorescence detection. J Chromatogr 224:168
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
D'Incalci, M., Farina, P., Sessa, C. et al. Pharmacokinetics of VP16-213 given by different administration methods. Cancer Chemother. Pharmacol. 7, 141–145 (1982). https://doi.org/10.1007/BF00254536
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00254536