Summary
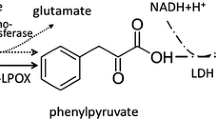
For production of l-phenylalanine the reductive amination of phenylpyruvate, catalyzed by phenylalanine-dehydrogenase was examined. To reach high levels and a sufficient stability of the inducible intracellular enzyme, growth conditions of Brevibacterium sp. are optimized. For continuous production of l-phenylalanine in an enzyme membrane reactor, the kinetic parameters of the partially purified enzyme are determined.
In continuous production a space time yield of 37.4 g l-Phe l-1 d-1 can be reached.
By means of the measured kinetic parameters and simultaneous calculation of the mass balances of all reaction components the behaviour of the reactor can be simulated. For certain conditions the multi-enzyme-system shows multiple steady-states.
Similar content being viewed by others
Abbreviations
- l-phe:
-
l-phenylalanine
- phepy:
-
phenylpyruvate
- PEG:
-
polyethylenglycol
- pheDH:
-
l-phenylalanine dehydrogenase
References
Asai T, Aida K, Oishi K (1959) On the enzymatic preparation of l-phenylalanine. J Gen Appl Microbiol 5:150–152
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bückmann AF, Kula M-R, Wichmann R, Wandrey C (1981) An efficient synthesis of high molecular weight NAD(H)-derivatives suitable for continuous operation with coenzyme depending enzyme systems. J Appl Biochem 3:301–315
Bulot E, Cooney CL (1985) Selective production of phenylalanine from phenylpyruvate using growing cells of Corynebacterium glutamicum. Biotechnol Lett 7:93–98
Choi YJ, Tribe DE (1982) Continuous production of l-phenylalanine using an Escherichia coli regulatory mutant. Biotechnol Lett 4:223–228
Crosby GA (1976) New Sweeteners. CRC Crit Rev Food Sci 7:297–323
Hummel W, Weiss N, Kula M-R (1984) Isolation and characterization of a bacterium possessing l-phenylalanine dehydrogenase activity. Arch Microbiol 137:47–52
Nakamichi K, Nabe K, Yamada S, Tosa T, Chibata I (1984) l-Phenylalanine formation from acetamidocinnamic acid by newly isolated bacteria. Appl Microbiol Biotechnol 19:100–105
Tokoro Y, Oshima K, Okii M, Yamaguchi K, Tanaka K, Kinoshita S (1970) Microbial production of l-phenylalanine from n-alkanes. Agr Biol Chem 34:1516–1521
Wichmann R, Wandrey C (1980) On-line determination of reaction rate versus substrate concentration for an enzymatically catalyzed reaction by means of a microcomputer. Enzyme Engineering 5:259–261
Wichmann R, Wandrey C, Bückmann AF, Kula M-R (1981) Continuous enzymatic transformation in an enzyme membrane reactor with simultaneous NAD(H) regeneration. Biotechnol Bioeng 23:2789–2802
Yamada S, Nabe K, Izuo N, Nakamichi K, Chibata I (1981) Production of l-phenylalanine from trans-cinnamic acid with Rhodotorula glutinis containing l-phenylalanine ammonia-lyase activity. Appl Environ Microbiol 42:773–778
Ziehr H, Kula M-R (1985) Isolation and characterization of a highly inducible l-aspartate-phenylpyruvate transaminase from Pseudomonas putida. J Biotechnol 3:19–31
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hummel, W., Schmidt, E., Wandrey, C. et al. l-Phenylalanine dehydrogenase from Brevibacterium sp. for production of l-phenylalanine by reductive amination of phenylpyruvate. Appl Microbiol Biotechnol 25, 175–185 (1986). https://doi.org/10.1007/BF00253645
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00253645