Abstract
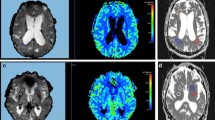
NPH can be reversible after cerebrospinal fluid (CSF) diversion. In the past no reliable criteria could be defined to predict the successful outcome of CSF shunting. Several authors demonstrated an increased cerebral blood flow after lumbar puncture in patients with NPH, indicating an underlying impairment of cerebral circulation autoregulation. 123I-AMP brain tomoscintigraphy was applied to 23 individuals with NPH before and after CSF drainage. Of these 23 patients, 10 underwent surgical shunting. The frontal and parietal hypoactive cortical pattern was present in NPH but not pathognomonic. Under stimulation of CSF pressure lowering, seven patients with improved outcome after shunting demonstrated an increase of cerebral perfusion in these areas, whereas a decrease of activity was found in three patients whose clinical status was unchanged after CSF diversion. This tomoscintigraphic test may be an interesting additional criterion for surgical admission.
Similar content being viewed by others
References
Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH (1965) Symptomatic occult hydrocephalus with “normal” cerebrospinal fluid pressure. N Engl J Med 273:117–126
Borgesen SE, Gjerris F (1982) The predictive value of conductance to outflow of CSF in normal pressure hydrocephalus. Brain 105:65–86
Di Chiro G, Arimitsu T, Brooks RA, Morgenthaler DG, Johnston GS, Jones AE, Keller MR (1979) CT profils of periventricular hypodensity in hydrocephalus and leucoencephalopathy. Radiology 130:611–666
Essen C von (1974) Effects of dopamine on the cerebral blood flow in the dog. Acta Neurol Scand 50:39–52
Fisher CM (1982) Hydrocephalus as a cause of disturbances of gait in the elderly. Neurology 32:1358–1363
Greitz T, Grepe AO, Kalmer MS, Lopez J (1969) Pre-and postoperative evaluation of cerebral blood flow in low-pressure hydrocephalus. J Neurosurg 31:644–651
Grubb RL, Raichle ME, Gado MH, Eichling JO, Hughes CP (1977) Cerebral blood flow, oxygen utilization and blood volume in dementia. Neurology (MN) 27:905–910
Hakim S, Adams RD (1965) The special clinical problem of symptomatic hydrocephalus with cerebrospinal fluid pressure. J Neurol Sci 2:307–327
Harbert JC, McCullough DC, Schellinger D (1977) Computed cranial tomography and radionuclide cisternography in hydrocephalus. Semin Nucl Med 7:197–200
Hartmann A, Alberti E, Lange D (1977) Effects of CSF drainage on CBF and CBV in subarachnoid haemorrhage and communicating of CSF pressure. J Neurol Neurosurg Psych 40:630–640
Huckman MC (1981) Normal pressure hydrocephalus: Evaluation of diagnostic and pronostic tests. AJNR 2:385–395
Hughes CP, Siegel BA, Coxe WS, Gado MH, Grubb RL, Coleman RE (1978) Adult idiopathic communicating hydrocephalus with and without shunting. J Neurol Neurosurg Pshych 41:961–971
Katzmann R, Hussey F (1970) A simple constant-infusion manometric test for measurement of CSF absorption. Neurology (MN) 20:534–544
Kuhl DE, Barrio JR, Huang SC, Selin C, Ackerman RF, Lear JL, Wu JL, Lin TH, Phelps ME (1982) Quantifying local cerebral blood flow by N-isopropyl-p-I-123 iodoamphetamine (IMP) tomography. J Nucl Med 23:196–203
Kushner M, Younkin D, Weinberger J, Hurtig H, Goldberg H, Reivich M (1984) Cerebral hemodynamics in the diagnosis of normal pressure hydrocephalus. Neurology 34:96–99
Lassen NA, Henriksen L, Holm S, Barry DI, Paulson OB, Worstrup S, Rapin J, Le Poncin Lafitte M, Moretti JL, Askienazy S, Raynaud C (1983) Cerebral blood flow tomography Xenon-133 compared to isopropyl-amphetamine iodine 123. J Nucl Med 24:17–23
Le May M, Hochberg FH (1979) Ventricular difference between hydrostatic hydrocephalus and hydrocephalus ex vacuo by computed tomography. Neuroradiology 17:191–195
Lying-Tunell U, Lidblad BS, Malmlund H, Persson B (1977) Cerebral blood flow and metabolic rate of oxygen, glucose, lactate, pyruvate, ketone bodies and amino acids in patients with NPH before and after shunting and in normal subjects. Acta Neurol Scand [Suppl] 56:338–339
Mathew NT, Meyer JS, Hartmann A, Ott EO (1975) Abnormal cerebrospinal fluid flow dynamics. Implications in diagnosis treatment, and prognosis in normal pressure hydrocephalus. Arch Neurol 32:657–664
Mchedlishivili GI, Nikolaishvili LS (1970) Evidence of a cholinergic nervous mechanism mediating the autoregulatory dilatation of the cerebral blood vessel. Pfluegers Arch 315:25–37
Meyer JS, Tachibana H, Hardenberg JP, Dowel RE, Kitawaga Y, Mortel KF (1984) Normal pressure hydrocephalus. Influences on cerebral hemodynamic and cerebrospinal fluid pressure chemical autoregulation. Surg Neurol 21:195–203
Moretti JL, Askienazy S, Raynaud C, Lassen NA, Sanabria E, Desplanches C, Rapin JR, Cesaro P, Marsault C, Barbizet J, Keravel Y, Fredoy D (1983) The N-isopropyl-p-iodoamphetamine I 123 in cerebral tomoscintigraphy. Ann Radiol 26:59–67
Penfield W, Elvidge AR (1932) Hydrocephalus and the atrophy of cerebral compression, In: Penfield W (ed) Cytology and cellular pathology of the nervous system. PB Hoeber, New York, p 1267
Price DL, Whitehouse PJ, Strubble RG, Clark AW, Coyle JT, Delong MR, Hedreen JC (1982) Basal forebrain cholinergic systems in Alzheimer's disease and related dementias. Neurosci. Comment 1:84–92
Salmon JH, Timperman AL (1971) Cerebral blood flow in posttraumatic encephalopathy: the effect of ventriculoatrial shunt. Neurology (MN) 21:33–42
Strump DA, Sires BP, Toole JF, McHenry LC, McWhorter JM (1983) Regional cerebral blood flow autoregulation and normal pressure hydrocephalus. Neurology [Suppl. 2] 33:182–187
Tamaki N, Kusonoki T, Wakabayashi T, Matsumoto S (1984) Cerebral hydrodynamics in normal pressure hydrocephalus. Evaluation by 133Xe inhalation method and dynamic CT study. J Neurosurg 61:510–514
Whitehouse PJ, Price DL, Struble RG, Clark AW, Koyle JT, Delong NR (1982) Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237–1239
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moretti, JL., Sergent, A., Louarn, F. et al. Cortical perfusion assessment with 123I-isopropyl amphetamine (123I-IAMP) in normal pressure hydrocepha lus (NPH). Eur J Nucl Med 14, 73–79 (1988). https://doi.org/10.1007/BF00253445
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00253445