Abstract
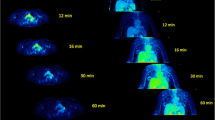
The membrane potential in cells can be estimated by electrophysiological techniques and biomedical methods using lipophilic cations labelled with 14C. However, these techniques cannot be applied to the human heart. In this study a lipophilic cation, triphenylmethylphosphonium (TPMP), was labelled with carbon-11 with the purpose of investigating its suitability for the estimation of membrane potential in vivo. A biodistribution study in mice and rats showed significant uptake of the cation in the heart a few minutes after IV injection which remained constant for 60 min. In vivo study by positron-emission tomography showed that after IV injection of 11C-TPMP in the dog, activity rose almost immediately in the myocardium and then remained constant for 60 min. A rapid injection of KCl (>40 mg/kg) 20 min after injection of 11C-TPMP led to an instantaneous fall in myocardial 11C-TPMP concentration. Membrane potential (ΔΨ), calculated from the TPMP distribution ratio between intracellular and plasma water space by the Nernst equation, was estimated at-148.1±6.0 mV for the dog heart. This value reflected both cell membrane potential and mitochondrial membrane potential and thus, the energy state of the myocardial cells.
Similar content being viewed by others
References
Azzone GF, Bragadin M, Pozzan T, Dell'Antone P (1976) Proton electrochemical potential in steady-state rat liver mitochondria. Biochim Biophys Acta 459:96–109
Bakeeva LE, Grinius LL, Jasiatis AA, Kuliene VV, Levitsky DO, Liberman EA, Severina II, Skulachev VP (1970) Conversion of biomembrane-produced energy into electric form. II. Intact mitochondria. Biochim Biophys Acta 216:13–21
Cohen LB, Salzberg BM, Davila HV, Ross WN, Landowne D, Waggoner AS, Wang CH (1974) Changes in axon fluorescence during activity: Molecular probe of membrane potential. J Membr Biol 19:1–36
Cohen SJ, Fozzard HA (1979) Intracellular K and Na activities in papilary muscle during inotropic interventions. Biophys J 25:144
Deutsch CJ, Holian A, Holian SK, Daniele RP, Wilson DF (1979) Transmembrane electrical and pH gradients across human erythrocytes and human peripheral lymphocytes. J Cell Physiol 99:79–94
Deutsch C, Erecinska M, Werrlein R, Silver IA (1979) Cellular energy metabolism, trans-plasma and trans-mitochondrial membrane petentials, and pH gradients in mouse neuroblastoma. Proc Natl Acad Sci USA 76:2175–2179
Friedman SM, Friedman CL (1962) Effects of ions on vascular smooth muscle. In: Hamilton WF (ed) Handbook of physiology: Circulation, vol 2. Waverly Press, Baltimore, pp 1135–1166
Glitsh HG (1982) Characteristics of active sodium transport in intact cardiac cells. In: Levy MN, Vassalle M (eds) Excitation and neuronal control of the heart. Waverly Press, Baltimore, pp 37–58
Gregg DE, Fisher LC (1962) Blood supply to the heart. In: Hamilton WF (ed) Handbook of physiology: Circulation, vol2. Waverly Press, Baltimore, pp 1517–1584
Grinius LL, Jaisaitis AA, Kadziauskas JP Liberman EA, Skulachev VP, Topali VP, Tsofina LM, Vladimirova MA (1970) Conversion of biomembrane-produced energy into electric form. I. Submitochondrial particles. Biochim Biophys Acta 216:1–12
Grinvald A, Manker A, Segal M (1982) Visualization of the spread of electrical activity in rat hippocampal slices by voltage-sensitive optical probes. J Physiol (London) 333:269–291
Grollman EF, Lee G, Ambesi-Impiombato FS, Meldolesi MF, Aloj SM, Coon HG, Kaback HR, Kohn LD (1977) Effects of thyrotropin on the thyroid cell membrane: Hyperpolarization induced by hormone-receptor interaction. Proc Natl Acad Sci USA 74:2352–2356
Guller B, Yipintsoi T, Orivis AL, Bassingthwaighte JB (1975) Estimation of capillary permeability by residue and outflow detection. Circ Res 37:359–378
Kamo N, Muratsugu M, Hongoh R, Kobatake Y (1979) Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphonylation potential in steady state. J Membr Biol 49:105–121
Kauppinen R (1983) Proton electrochemical potential of the inner mitochondrial membrane in isolated perfused rat hearts as measured by exogenous probes. Biochim Biophys Acta 725:131–137
Kauppinen RA, Hassinen IE (1984) Monitoring of mitochondrial membrane potential in isolated perfused rat heart. Am J Physiol 247:508–516
Milligan G, Strange PG (1984) Use of [3H]-triphenylmethylphosphonium cation for estimating membrane potential in neuroblastoma cells. J neurochem 43:1515–1521
Mitchell P, Moyle J (1969) Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem 7:471–484
Page E, Page EG (1968) Distribution of ions and water between tissue compartments in the perfused left ventricle of the rat heart. Circ Res 22:435–446
Polomeni PI (1974) Extracellular space and ionic distribution in rat ventricle. Am J Physiol 227:676–683
Ritchie RJ (1984) A critical assessment of the use of lipophilic cations as membrane potential probes. Prog Biophys Mol Biol 43:1–32
Smith QR, Pershing LK, Johanson CE (1981) A comparative analysis of extracellular fluid volume of several tissues as determined by six different markers. Life Sci 29:449–456
Sperelakis N (1979) Origin of the cardiac resting potential. In: Berne RM, Sperelakis W (eds) Handbook of physiology: The cardiovascular system, vol 1. Waverly Press, Baltimore, pp 187–267
Syrota A, Samson Y, Boullais C, Wajnberg P, Loc'h, Crouzel C, Mazière B, Soussaline F, Baron JC (1985) Tomographic mapping of brain intracellular pH and extracellular water space in stroke patients. J Cereb Blood Flow Metab 5:358–368
Waggoner AS (1979) Dye indicators of membrane potential. Annu Rev Biophys Bioeng 8:47–68
Walker JL, Ladle RO (1973) Frog heart intracellular potassium activities measured with potassium microelectrode. Am J Physiol 225:263–267
Yipintsoi T, Scanlon PD, Bassingthwaighte JB (1972) Density and water content of dog ventricular myocardium. Proc Soc Exp Biol Med 141:1032–1035
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fukuda, H., Syrota, A., Charbonneau, P. et al. Use of 11C-triphenylmethylphosphonium for the evaluation of membrane potential in the heart by positron-emission tomography. Eur J Nucl Med 11, 478–483 (1986). https://doi.org/10.1007/BF00252793
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00252793