Abstract
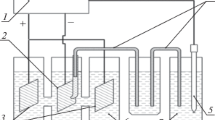
The production of aluminium of primary quality from scrap by electrorefining may become an option of strategic importance. Two important requirements are: (i) substantial energy savings compared to electrowinning, and (ii) easy recycling of alloying elements and molten electrolyte without ecological hazards. The use of molten chloride instead of fluoride electrolytes is preferred as emissions are low, purification of contaminated salts in aqueous solution is easy and oxide ceramic materials for cells and diaphragms can be used. The measurement of formal potentials of most important alloying elements shows that only manganese should be expected to cause trouble in electrorefining of aluminium scrap from alkali chloride melts. Preparative batch refining experiments show that all these alloying metals can be easily separated; manganese is very likely because its activity in aluminium is decreased by alloying or compound formation in aluminium. Results with cells divided by alumina diaphragms show that energy consumptions can be kept below 5 kWh (kg Al)−1.
Similar content being viewed by others
References
J. P. Pemsler and M. Dempsey, ‘Electrorefining of Aluminium’, Gouv. Rep. Announce, NSF/CPE-81012, B.P. 81-243693 (1981).
W. Hirt, H. K. Johnson, S. Wilkening and S. Winkhaus, ‘Aluminium and Magnesium’, in H. Harnisch, R. Steiner and K. Winnacker Hergb., ‘Chemishe Techno logie', vol. 4, Carl Hauser Verlag, München, Wien (1986) pp. 235ff.
T. A. Sullivan and R. L. De Beauchamp, ‘Recovery of aluminium, base and precious metal from electronic scrap’, Bureaux des Mines, RI-7617, BP-2090 (1971).
A. Klemm, ‘Ionic mobilities’ in ‘Advances of Molten Salt Chemistry’, vol. 6 (edited by G. Mamantov, C. B. Mamantov and J. Braunstein), Elsevier, Amsterdam (1987).
D. J. Fray, British Patent Application 130 821 (1986).
H. Linga, H. Øye and K. Motzfeld, Ber. Bunsenges. Phys. Chem. 85 (1981) 1132.
R. Tunold and R. Ødegard, ‘Kinetics of the deposition of aluminium from chloride melts’, in Proceedings Vth International Symposium on Molten Salts (edited by M. L. Saboungi, K. Johnson, D. S. Newman and D. Inman), Electrochem. Soc. Proc. vol., Proceedings Volume 86-1 (1986) pp. 408ff.
R. Ødegard, A. Bjørgum, A. Sterten, J. Thonstad and R. Tunold, Electrochim. Acta 27 (1982) 1595.
T. Takayama, H. Seto, J. Uchida and S. Hinotani, J. Appl. Electrochem. 24 (1994) 131.
W. Biltz, G. Rohloffs and H. U. v. Vogel, Z. anorg. allgem. Chemie 220 (1934) 113.
Aluminium-Gold, in ‘Constitution of Binary Alloys’ (edited by M. Hansen and K. Anderko), McGraw-Hill, New York (1958) pp. 68ff.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schwarz, V., Wendt, H. Electrorefining of aluminium scrap from chloride melts. J Appl Electrochem 25, 34–40 (1995). https://doi.org/10.1007/BF00251262
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00251262