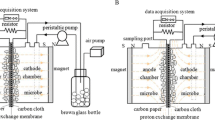
Abstract
A broad overview of research on the effects of imposed magnetic fields on electrolytic processes is given. As well as modelling of mass transfer in magnetoelectrolytic cells, the effect of magnetic fields on reaction kinetics is discussed. Interactions of an imposed magnetic field with cathodic crystallization and anodic dissolution behaviour of metals are also treated. These topics are described from a practical point of view.
Similar content being viewed by others
Abbreviations
- α1, α2:
-
regression parameters (-)
- B :
-
magnetic field flux density vector (T)
- c :
-
concentration (mol m−3)
- c ∞ :
-
bulk concentration (mol m−3)
- D :
-
diffusion coefficient (m2 s−1)
- d e :
-
diameter of rotating disc electrode (m)
- E :
-
electric field strength vector (V m−1)
- E i :
-
induced electric field strength vector (V m−1)
- E g :
-
electrostatic field strength vector (V m−1)
- F :
-
force vector (N)
- F :
-
Faraday constant (C mol−1)
- H :
-
magnetic field strength vector (A m−1)
- i :
-
current density (A m−2)
- i L :
-
limiting current density (A m−2)
- i 0L :
-
limiting current density without applied magnetic field (A m−2)
- I :
-
current (A)
- I L :
-
limiting current (A)
- j :
-
current density vector (A m−2)
- K :
-
reaction equilibrium constant
- k :
-
reaction velocity constant
- k b :
-
Boltzmann constant (J K−1)
- m 1, m 2 :
-
regression parameters (-)
- n :
-
charge transfer number (-)
- q :
-
charge on a particle (C)
- R :
-
gas constant (J mol−1 K−1)
- T :
-
temperature (K)
- t :
-
time (s)
- V :
-
electrostatic potential (V)
- v :
-
particle velocity vector (m s−1)
- α:
-
transfer coefficient (−)
- γ:
-
velocity gradient (s−1)
- ΔMS :
-
potential difference between metal phase and point just inside electrolyte phase (OHP)
- σ:
-
diffusion layer thickness (m)
- δ0 :
-
hydrodynamic boundary layer thickness without applied magnetic field (m)
- ϱ:
-
density (kg m−3)
- σ:
-
electrolyte conductivity (Ω−1 m−1)
- μ:
-
magnetic permeability (V s A−1 m−1)
- ν:
-
kinematic viscosity (m2 s−1)
- ω:
-
vorticity
References
T. Z. Fahidy, J. Appl. Electrochem. 13 (1983) 553.
J. A. Shercliff, ‘Magnetohydrodynamics’, Pergamon Press, Oxford (1965).
P. Robert, ‘Electric and mechanical properties of materials’, Artec House, Norwood U.K. (1988).
T. Z. Fahidy, Electrochim. Acta 18 (1973) 607.
R. N. O'Brien and K. S. V. Santhanam, Electrochim. Acta 32 (1987) 1679.
T. Z. Fahidy and T. S. Rutherford, J. Appl. Electrochem. 10 (1980) 481.
M. S. Quraishi and T. Z. Fahidy, Electrochim. Acta 25 (1980) 591.
M.I. Ismail and T. Z. Fahidy, Can. J. Chem. Eng. 58 (1980) 505.
T. Z. Fahidy, Chem. Eng. J. 17 (1979) 245.
Idem, ibid., 7 (1974) 21.
S. Mohanta and T. Z. Fahidy, Can. J. Chem. Eng. 50 (1972) 248.
J. P. Chopart, J. Douglade, P. Fricoteaux and A. Olivier, Electrochim. Acta 36 (1991) 459.
O. Aaboubi, J. P. Chopart, J. Douglade, A. Olivier, C. Gabrielli and B. Tribollet, J. Electrochem. Soc. 137 (1990) 1796.
S. Mohanta and T. Z. Fahidy, J. Appl. Electrochem. 8 (1978) 5.
Idem, ibid., 8 (1978) 265.
T. Z. Fahidy, Electrochim. Acta 35 (1990) 929.
S. D. Lympany, J. W. Evans and R. Moreau, Proceedings of the International Union of Theoretical Applied Mechanics, The Metals Society, London (1984) p. 15.
J. Dash, US Patent 4 666 568 (1987).
K. Higashitani, K. Okuhara and S. Hatade, J. Coll. Interf. Sci. 152 (1992) 125.
Matsushita Electric Works Ltd., Japanese Patent 59 31 894 (1984).
Idem, Chem. Abstr. 101 (1984) 45482.
N. J. Turro, M. F. Chow, C. J. Chung and C. H. Tung, J. Am. Chem. Soc. 105 (1983) 1572.
Y. Tanimoto, H. Udagawa and M. Itch, J. Phys. Chem. 87 (1983) 724.
N. J. Turro, M. F. Chow, C. J. Chung and B. Kracutler, J. Am. Chem. Soc. 103 (1981) 3886.
P. Lin, J. Peng, B. Hou and Y. Fu, J. Phys. Chem. 97 (1993) 1471.
G. Zadel, C. Eisenbraun, G. J. Wolff and E. Bretmaier, Angew. Chem. Int. Ed. Engl. 33 (1994) 454.
I. Ohno, Electrodep. Surf. Treat. 3 (1975) 213.
J. K. Kelly, J. Electrochem. Soc. 124 (1977) 987.
H. Zhang and G. Zhang, Chem. Abstr. 115 (1991) 217159.
C. Iwakura, M. Kitayama, T. Edamoto and H. Tamura, Electrochim. Acta 30 (1985) 747.
P. Fricoteaux, A. Olivier and R. Delmas, J. Electrochem. Soc. 139 (1992) 1096.
A. Olivier and T. Z. Fahidy, J. Appl. Electrochem. 12 (1982) 417.
M. I. Ismail and T. Z. Fahidy, KFAS Proc. Ser. 2 (Corrosion) (1984) 253.
A. Chiba, K. Ogura, T. Ogawa and T. Yamashita, Chem. Abstr. 111 (1989) 183087.
T. Yamashita and A. Chiba, Chem. Abstr. 111 (1989) 183086.
I. Mogi, G. Kodo and Y. Nakagawa, Bull. Chem. Soc. Jpn. 63 (1990) 1871.
A. Chiba, T. Niimi, H. Kitayama and T. Ogawa, Surf. Coat. Tech. 29 (1986) 347.
A. Danilyuk, V. Kurmashev and A. Matyushkov, Thin Solid Films 189 (1990) 247.
R. N. O'Brien and K. S. V. Santhanam, J. Appl. Electrochem. 20 (1990) 781.
J. Dash and W. W. King, J. Electrochem. Soc. 119 (1972) 51.
A. Chiba, K. Kitamura and T. Ogawa, Surf. Coat. Tech. 27 (1986) 83.
M. I. Ismail and T. Z. Fahidy, Metall (Berlin) 39 (1984) 118.
R. N. O'Brien and K. S. V. Santhanam, Can. J. Chem. 65 (1987) 2009.
A. Chiba and T. Ogawa, Chem. Abstr. 108 (1988) 175888.
M. Perakh, J. Electrochem. Soc. 122 (1975) 1260.
K. Nemes, Tagungsband-Kammer der Technik Suhl 83 (1987) 144.
S. Mohanta and T. Z. Fahidy, Can. J. Chem. Eng. 50 (1972) 434.
Idem, Electrochim. Acta 19 (1974) 771.
Ling Yang, J. Electrochem. Soc. 101 (1954) 456.
M. I. Ismail and T. Z. Fahidy, J. Appl. Electrochem. 11 (1981) 543.
Z. H. Gu and T. Z. Fahidy, Can. J. Chem. Eng. 70 (1992) 127.
Z. H. Gu, J. Chen and T. Z. Fahidy, Electrochim. Acta 38 (1993) 2631.
Z. H. Gu, J. Chen, A. Olivier and T. Z. Fahidy, J. Electrochem. Soc. 140 (1993) 408.
Z. H. Gu, T. Z. Fahidy and J. P. Chopart, Electrochim. Acta 37 (1992) 97.
P. Fricoteaux, Z. H. Gu and T. Z. Fahidy, J. Electroanal. Chem. 324 (1992) 161.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tacken, R.A., Janssen, L.J.J. Applications of magnetoelectrolysis. J Appl Electrochem 25, 1–5 (1995). https://doi.org/10.1007/BF00251257
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00251257