Abstract
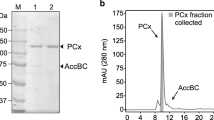
Cell-free extracts of Pseudomonas sp. strains KB 740 and K 172 both contained high levels of glutaryl-CoA dehydrogenase when grown anaerobically on benzoate or other aromatic compounds and with nitrate as electron acceptor. These aromatic compounds have in common benzoyl-CoA as the central aromatic intermediate of anerobic metabolism. The enzymatic activity was almost absent in cells grown aerobically on benzoate regardless whether nitrate was present. Glutaryl-CoA dehydrogenase activity was also detected in cell-free extracts of Rhodopseudomonas, Rhodomicrobium and Rhodocyclus after phototrophic growth on benzoate. Parallel to the induction of glutaryl-CoA dehydrogenase as measured with ferricenium ion as electron acceptor, an about equally high glutaconyl-CoA decarboxylase activity was detected in cell-free extracts. The latter activity was measured with the NAD-dependent assay, as described for the biotin-containing sodium ion pump glutaconyl-CoA decarboxylase from glutamate fermenting bacteria. Glutaryl-CoA dehydrogenase was purified to homogeneity from both Pseudomonas strains. The enzymes catalyse the decarboxylation of glutaconyl-CoA at about the same rate as the oxidative decarboxylation of glutaryl-CoA. The green enzymes are homotetramers (m=170 kDa) and contain 1 mol FAD per subunit. No inhibition was observed with avidin indicating the absence of biotin. The N-terminal sequences of the enzymes from both strains are similar (65%).
Similar content being viewed by others
References
Beatrix B, Bendrat K, Rospert S, Buckel W (1990) The biotindependent sodium ion pump glutaconyl-CoA decarboxylase from Fusobacterium nucleatum (subsp. nucleatum). Comparison with glutaconyl-CoA decarboxylases from Gram-positive bacteria. Arch Microbiol 154: 362–369
Besrat A, Polan CE, Henderson LM (1969) Mammalian metabolism of glutaric acid. J Biol Chem 244: 1461–1467
Blakley ER (1978) The microbial degradation of cyclohexanecarboxylic acid by a β-oxidation pathway with simultaneous induction to the utilisation of benzoate, Can J Microbiol 24: 847–855
Braun K, Gibson DT (1984) Anaerobic degradation of 2-aminobenzoate (anthranilic acid) by denitrifying bacteria. Appl Environ Microbiol 48: 102–107
Buckel W (1986) Biotin-dependent decarboxylase as bacterial sodium pumps: purification and reconstitution of glutaconyl-CoA decarboxylase from Acidaminococcus fermentans. In: Fleischer S, Fleischer B (eds) Methods in enzymology, vol 125, Academic Press, New York London, pp 547–558
Buckel W, Dorn U, Semmler R (1981) Glutaconate CoA-transferase from Acidaminococcus fermentans. Eur J Biochem 118: 315–321
Byron CM, Stankovich MT, Husain M (1990) Spectral and electrochemical properties of glutaryl-CoA dehydrogenase from Paracoccus denitrificans. Biochemistry 29: 3691–3700
Evans WC, Fuchs G (1988) Anaerobic degradation of aromatics. Annu Rev Microbiol 42: 289–317
Gomes B, Fendrich G, Abeles RH (1981) Mechanism of action of glutaryl-CoA and butyryl-CoA dehydrogenase. Biochemistry 20: 1481–1490
Goodman SI, Markey SP, Moe PG, Miles BS, Teng CC (1975) Glutaric aciduria: a “new” disorder of amino acid metabolism. Biochem Med 12: 12–21
Harwood CS, Gibson J (1986) Uptake of benzoate by Rhodopseudomonas palustris grown anaerobically in light. J Bacteriol 165: 504–509
Haworth JC, Booth FA, Chudley AE, Degroot GW, Dilling LA, Goodman SI, Greenberg CR, Mallroy CJ, McClarty BM, Seshia SS, Seargeant LE (1991) Phenotypic variability in glutaric aciduria type I: report of fourteen cases in five Canadian Indian kindreds. J Pediatr 118: 52–58
Husain M, Steenkamp DJ (1985) Partial purification and characterization of glutaryl-coenzyme A dehydrogenase, electron transfer flavoprotein, and electron transfer flavoprotein-Q oxidoreductase from Paracoccus denitrificans. J Bacteriol 163: 709–715
Koch J, Fuchs G (1992) Enzymatic reduction of benzoyl-CoA to alicyclic compounds, a key reaction in anaerobic aromatic metabolism. Eur J Biochem 205: 195–202
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Lehman TC, Hale DE, Bhala A, Thorpe C (1990) An acyl-coenzyme A dehydrogenase assay utilizing ferricenium ion. Anal Biochem 186: 280–284
Lochmeyer C, Koch J, Fuchs G (1992) Anaerobic degradation of 2-aminobenzoic acid (anthranilic acid) via benzoyl-coenzyme A (CoA) and cyclohex-1-enecarboxyl-CoA in a denitrifying bacterium. J Bacteriol 174: 3621–3628
Michel C, Buckel W, Linder D (1991) Purification of the coenzyme B12-containing 2-methyleneglutarate mutase from Clostridium barkeri by high-performance liquid chromatography. J Chromatogr 587: 93–99
Numa S, Ishimura Y, Nakazawa T, Okazaki T, Hayaishi O (1964) Enzymic studies on the metabolism of glutarate in Pseudomonas. J Biol Chem 239: 3915–3926
Pfennig N (1978) Rhodocyclus purpureus gen. nov. and sp. nov., a ring shaped, vitamin B12-requiring member of the family Rhodospirillaceae. Int J Syst Bacteriol 28: 283–288
Pfennig N (1989) Genus I. Chromatium Perty 1852. In: Stanley JT, Bryant MP, Pfennig N, Holt JG (eds) Bergey's manual of systematic bacteriology, vol 3. Williams and Wilkins, Baltimore, pp 1639–1643
Schink B, Brune A, Schnell S (1992) Anaerobic degradation of aromatic compounds. In: Winkelmann G (ed) Microbial degradation of natural products. VCH Verlagsgesellschaft, Weinheim
Simon EJ, Shemin D (1953) The preparation of S-succinyl-CoA. J Am Chem Soc 75: 2520–2520
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85
Stanier Y, Ingraham JL, Wheelis ML, Painter PR (1987) General microbiology 5th ed. Macmillan Education LTD, Basingstoke, UK, p 406
Stokke O, Goodman SI, Thompson JA, Miles BS (1975) Glutaric aciduria; presence of glutaconic and β-hydroxyglutaric acids in urine. Biochem Med 12: 386–391
Tayeh MA, Madigan MT (1987) Malate dehydrogenase in phototrophic purple bacteria: purification, molecular weight, and quaternary structure. J Bacteriol 169: 4196–4202
Tschech A, Fuchs G (1987) Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch Microbiol 148: 213–217
Whitaker JR, Granum PE (1980) An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem 109: 156–159
Williamson G, Engel PC, Mizzer JP, Thorpe C, Massey V (1982) Evidence that the greening ligand in native butyryl-CoA dehydrogenase is a CoA persulfide. J Biol Chem 257: 4314–4320
Zehnder ABJ, Wuhrmann K (1976) Titanium(III)citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science 194: 1165–116
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Härtel, U., Eckel, E., Koch, J. et al. Purification of glutaryl-CoA dehydrogenase from Pseudomonas sp., an enzyme involved in the anaerobic degradation of benzoate. Arch. Microbiol. 159, 174–181 (1993). https://doi.org/10.1007/BF00250279
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00250279