Abstract
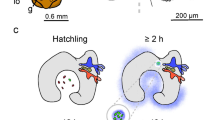
A pure culture of the luminous bacterium Vibrio fischeri is maintained in the light-emitting organ of the sepiolid squid Euprymna scolopes. When the juvenile squid emerges from its egg it is symbiont-free and, because bioluminescence is part of an anti-predatory behavior, therefore must obtain a bacterial inoculum from the surrounding environment. We document here the kinetics of the process by which newly hatched juvenile squids become infected by symbiosis-competent V. fischeri. When placed in seawater containing as few as 240 colony-forming-units (CFU) per ml, the juvenile became detectably bioluminescent within a few hours. Colonization of the nascent light organ was initiated with as few as 1 to 10 bacteria, which rapidly began to grow at an exponential rate until they reached a population size of approximately 105 cells by 12 h after the initial infection. Subsequently, the number of bacteria in the established symbiosis was maintained essentially constant by a combination of both a >20-fold reduction in bacterial growth rate, and an expulsion of excess bacteria into the surrounding seawater. While V. fischeri cells are normally flagellated and motile, these bacteria did not elaborate these appendages once the symbiosis was established; however, they quickly began to synthesize flagella when they were removed from the light organ environment. Thus, two important biological characteristics, growth rate and flagellation, were modulated during establishment of the association, perhaps as part of a coordinated series of symbiotic responses.
Similar content being viewed by others
References
Akerley BJ, Monack DM, Falkow S, Miller JF (1992) The bvg locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J Bacteriol 174: 980–990
Allen RD, Baumann P (1971) Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J Bacteriol 107: 295–302
Amy PS, Pauling C, Morita RJ (1983) Recovery from nutrient starvation by a marine Vibrio sp. Appl Environ Microbiol 46: 1685–1690
Baker RM, Singleton FL, Hood MA (1983) Effects of nutrient deprivation on Vibrio cholerae. Appl Environ Microbiol 46: 930–940
Belas R, Simon M, Silverman M (1986) Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol 167: 210–218
Boettcher KJ, Ruby EG (1990) Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol 172: 3701–3706
Buchmeier NA, Heffron F (1990) Induction of Salmonella stress proteins upon infection of macrophages. Science 248: 730–732
Colley NJ, Trench RK (1985) Cellular events in the reestablishment of a symbiosis between a marine dinoflagellate and a coelenterate. Cell Tissue Res 239: 93–103
Dolan KM, Greenberg EP (1992) Evidence that GroEL, not σM32 is involved in transcriptional regulation of the Vibrio fischeri luminescence genes in Escherichia coli. J Bacteriol 174: 5132–5135
Dunlap PV (1984) Physiological and morphological state of the symbiont bacteria from light organs of ponyfish. Biol Bull 167: 410–425
Dunlap PV (1991) Organization and regulation of bacterial luminescence genes. Photochem Photobiol 54: 1157–1170
Dunlap PV, McFall-Ngai M (1987) Initiation and control of the bioluminescent symbiosis between Photobacterium leiognathi and leiognathid fish. Ann NY Acad Sci 503: 269–283
Felbeck H, Distel D (1991) Prokaryotic symbionts of marine invertebrates. In: Balows A, Truper HG, Dworkin M, Harder W, Schleifer K-H (eds). The prokaryotes, a handbook on the biology of bacteria: ecophysiology, isolation, identification, applications. 2nd edn. Springer, Berlin Heidelberg New York, pp 3892–3906
Fiala-Medioni A, Felbeck H, Childress JJ, Fisher CR, Vetter RD (1990) Lysosomic resorption of bacterial symbionts in deep-sea bivalves. In: Nardon P, Gianinazzi-Pearson V, Grenier AM, Margulis L, Smith DC (eds) Endocytobiology IV. Institut National de la Recherche Agronomique, Paris, pp 335–338
Gray KM, Greenberg EP (1992) Physical and functional maps of the luminescence gene cluster in an autoinducer-deficient Vibrio fischeri isolated from a squid light organ. J Bacteriol 174: 4384–4390
Hastings JW, Weber G (1963) Total quantum flux of isotropic sources. J Opt Soc Am 53: 1410–1415
Haygood MG, Tebo BM, Nealson KH (1984) Luminous bacteria of a monocentrid fish (Monocentris japonicus) and two anomolopid fishes (Photoblepharon palpebratus and Kryptophanaron alfredi): population sizes and growth within the light organs, and rates of release into seawater. Mar Biol 78: 249–254
Hobbie JE, Daley RJ, Jasper S (1977) Use of Nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33: 1225–1228
Jenkins DE, Auger EA, Matin A (1991) Role of RpoH, a heat shock regulator protein, in Escherichia coli carbon starvation protein synthesis and survival. J Bacteriol 173: 1992–1996
Kaplan H, Greenberg EP (1985) Diffusion of autoinducer is involved in the regulation of the Vibrio fischeri luminescence system. J Bacteriol 163: 1210–1214
Kishitani T (1928) Über das leuchtorgan von Euprymna morsei Verrill und die symbiotischen leuchtbakterien. Proc Imp Acad 4: 306–309
Lee K-H, Ruby EG (1992) Detection of the light organ symbiont, Vibrio fischeri, in Hawaiian seawater by using lux gene probes. Appl Environ Microbiol 58: 942–947
Long SR (1989) Rhizobium-legume nodulation: life together in the underground. Cell 56: 203–214
Malmcrona-Friberg K, Goodman A, Kjelleberg S (1990) Chemotactic responses of marine Vibrio sp. strain S14 (CCUG 15956) to low-molecular-weight substances under starvation and recovery conditions. Appl Environ Microbiol 56: 3699–3704
Matin A, Auger EA, Blum PH, Schultz JE (1989) Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol 29: 293–316
Mekalanos JJ (1992) Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol 174: 1–7
McCarter LL, Silverman M (1987) Phosphate regulation of gene expression in Vibrio parahaemolyticus. J Bacteriol 169: 3441–3449
McCarter LL, Silverman M (1989) Iron regulation of swarmer cell differentiation of Vibrio parahaemolyticus. J Bacteriol 171: 731–736
McFall-Ngai MJ, Montgomery M (1990) The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda: Sepiolidae). Biol Bull 179: 332–339
McFall-Ngai MJ, Ruby EG (1991) Symbiont recognition and subsequent morphogenesis as early events in an animal-bacteria mutualism. Science 254: 1491–1494
Montgomery M, McFall-Ngai MJ (1991) The influence of bacterial symbionts on early morphogenesis of the light organ of the sepiolid squid Euprymna scolopes. Am Zool 31: 15A
Nardon P, Grenier AM (1989) Endocytobiosis in coleoptera: biological, biochemical, and genetic aspects. In: Schwemmler W, Gassner G (eds) Insect endocytobiosis: morphology, physiology, genetics, evolution. CRC Press, Boca Raton, Florida, pp 175–216
Nealson KH (1977) Autoinduction of bacterial luciferase: occurrence, mechanism and significance, Arch Microbiol 112: 73–79
Nealson KH, Hastings JW (1991) The luminous bacteria, In: Balows A, Truper HG, Dworkin M, Harder W, Schleifer K-H (eds) The prokaryotes, a handbook on the biology of bacteria: ecophysiology, isolation, identification, applications. 2nd edn. Springer, Berlin Heidelberg New York, pp 625–639
Nealson KH, Schmidt TM, Bleakley B (1990) Physiology and biochemistry of Xenorhabdus. In: Gaugler R, Kaya H (eds) Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, Florida, pp 271–284
Neidhardt FC (1987) Multigene systems and regulons. In: Neidhardt FC, Ingraham JL, Low KB, Magasanik B, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella typhimurium: cellular molecular biology. American Society for Microbiology, Washington, DC, pp 1313–1317
Ohtaka C, Nakamura H, Ishikawa H (1992) Structures of chaperonins from an intracellular symbiont and their functional expression in Escherichia coli groE mutants. J Bacteriol 174: 1869–1874
Ruby EG, McFall-Ngai MJ (1992) A squid that glows in the night: development of an animal/bacterial mutualism. J Bacteriol 174: 4865–4870
Smith DC, Douglas AE (1987) The biology of symbiosis. Edward Arnold, London
Sokal PA, Woods DE (1984) Relationship of iron and extracellular virulence factors to Pseudomonas aeroginosa lung infections. J Med Microbiol 18: 125–133
Sutton WD, Pankhurst CE, Craig AS (1981) The Rhizobium baceroid state. In: Giles KI, Atherly AG (eds) Biology of the Rhizobiaceae. Academic Press, New York, pp 149–177
Tebo BM, Linthicum DS, Nealson KH (1979) Luminous bacteria and light-emitting fish: ultrastructure of the symbiosis. Biosystems 11: 269–280
Vasse J, DeBilly F, Camut S, Truchet G (1990) Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol 172: 4295–4306
Vincent JM (1980) Factors controlling the legume-Rhizobium symbiosis. In: Newton WE, Orme-Johnson WH (eds) Nitrogen fixation, vol. II. University Park Press, Baltimore, pp 103–109
Waterbury JB, Calloway CB, Turner RD (1983) A cellulolytic nitrogen-fixing bacterium cultured from the gland of Deshayes in shipworms (Bivalva: Teredinidae). Science 221: 1401–1403
Wei SL, Young RE (1989) Development of symbiotic bacterial bioluminescence in a nearshore cephalopod, Euprymna scolopes. Mar Biol 103: 541–546
Wei Z-M, Sneath BJ, Beer SV (1992) Expression of Erwinia amylovora hrp genes in response to environmental signals. J Bacteriol 174: 1875–1882
Yokota T, Gots JS (1970) Requirement of adenosine 3′,5′-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol 103: 513–516
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ruby, E.G., Asato, L.M. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol. 159, 160–167 (1993). https://doi.org/10.1007/BF00250277
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00250277