Summary
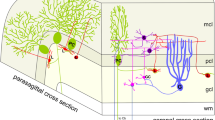
The distributions of taurine-like and GABA-like immunoreactivities in the rat cerebellum were compared by analysis of consecutive semithin and ultrathin sections, postembedding labeled with the peroxidase-antiperoxidase technique or with an indirect immunogold procedure, respectively. Taurine-like immunoreactivity was selectively enriched in Purkinje cell bodies, dendrites and spines, and boutons in the cerebellar nuclei exhibiting ultrastructural features typical of Purkinje cell terminals. The stellate and basket cell bodies and terminals were very weakly labeled. A computer assisted quantitative assessment of the net immunogold labeling revealed that the mean gold particle density in the Purkinje cell terminals was about 70% higher than that in the Purkinje cell dendrites, and about 14 times higher than that in the stellate/basket cell terminals in the molecular layer. Stellate, basket and Purkinje cell terminals emerged as intensely immunoreactive in adjacent sections processed with an antiserum against conjugated GABA. These findings indicate, contrary to recent electrophysiological data, that GABA is a more likely transmitter candidate than taurine in the stellate cells. The apparent colocalization of GABA and taurine in the terminals of Purkinje cells raises the possibility that these terminals are capable of releasing two different inhibitory amino acids.
Similar content being viewed by others
References
Andersen P, Eccles JC, Voorhoeve PE (1964) Postsynaptic inhibition of cerebellar Purkinje cells. J Neurophysiol 27: 1139–1153
Campistron G, Geffard M, Buijs RM (1986) Immunological approach to the detection of taurine and immunocytochemical results. J Neurochem 46: 862–868
Cavallini D, Scandurra R, Dupré S, Santoro L, Barra D (1976) A new pathway of taurine biosynthesis. Physiol Chem Physics 8: 157–160
Chan-Palay V (1977) Cerebellar dentate nucleus. Organization, cytology and transmitters. Springer, Berlin Heidelberg New York
Chan-Palay V, Palay SL, Wu J-Y (1982a) Sagittal cerebellar microbands of taurine neurons: immunocytochemical demonstration by using antibodies against the taurine-synthesizing enzyme cysteine sulfinic acid decarboxylase. Proc Natl Acad Sci USA 79: 4221–4225
Chan-Palay V, Lin C-T, Palay S, Yamamoto M, Wu J-Y (1982b) Taurine in the mammalin cerebellum: demonstration by autoradiography with [3H]taurine and immunocytochemistry with antibodies against the taurine-synthesizing enzyme, cysteine-sulfinic acid decarboxylase. Proc Natl Acad Sci USA 79: 2695–2699
Collins CGS (1972) GABA-2-oxoglutarate transaminase, glutamate decarboxylase and the half-life of GABA in different areas of rat brain. Biochem Pharmacol 21: 2849–2858
Curtis DR, Felix D (1971) The effect of bicuculline upon synaptic inhibition in the cerebral and cerebellar cortices of the cat. Brain Res 34: 301–321
Curtis DR, Duggan AW, Felix D (1970) GABA and inhibition of Deiters' neurones. Brain Res 23: 117–120
Eccles JC, Ito M, Szentágothai J (1967) The cerebellum as a neuronal machine. Springer, New York Heidelberg Berlin
Fonnum F, Storm-Mathisen J, Walberg F (1970) Glutamate decarboxylase in inhibitory neurons. A study of the enzyme in Purkinje cell axons and boutons in the cat. Brain Res 20: 259–275
Gabbott PLA, Somogyi J, Stewart MG, Hamori J (1986) GABAimmunoreactive neurons in the rat cerebellum: a light and electron microscopic study. J Comp Neurol 251: 474–490
Hodgson AJ, Penke B, Erdei A, Chubb IW, Somogyi P (1985) Antiserum to γ-aminobutyric acid. I. Production and characterisation using a new model system. J Histochem Cytochem 33: 229–239
Hökfelt T, Ljungdahl Å (1970) Cellular localization of labeled gamma-aminobutyric acid (3H-GABA) in rat cerebellar cortex: an autoradiographic study. Brain Res 22: 391–396
Huxtable RJ (1981) Sources and turnover rates of taurine in nursing and weaned rat pups. J Nutr 111: 1275–1286
Ito M, Yoshida M, Obata K, Kawai N, Udo M (1970) Inhibitory control of intracerebellar nuclei by the Purkinje cell axons. Exp Brain Res 10: 64–80
Kuriyama K, Ida S, Nishimura C, Ohkuma S (1983) Distribution and function of taurine in nervous tissues: an introductory review. In: Kuriyama K, Huxtable R, Iwata H (eds) Sulfur amino acids: biochemical and clinical aspects. Liss, New York, pp 127–140
Madsen S, Ottersen OP, Storm-Mathisen J (1985) Immunocytochemical visualization of taurine: neuronal localization in the rat cerebellum. Neurosci Lett 60: 255–260
McLaughlin BJ, Wood JG, Saito K, Barber R, Vaughn JE, Roberts E, Wu JY (1974) The fine structural localization of glutamate decarboxylase in synaptic terminals of rodent cerebellum. Brain Res 76: 377–391
Mugnaini E, Oertel WH (1985) An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunocytochemistry. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, Vol 4. Elsevier, Amsterdam, pp 436–608
Namima M, Okamoto K, Sakai Y (1983) Modulatory action of taurine on the release of GABA in cerebellar slices of the guinea pig. J Neurochem 40: 1–9
Obata K, Takeda K (1969) Release of gamma-aminobutyric acid into the fourth ventricle induced by stimulation of the cat's cerebellum. J Neurochem 16: 1043–1047
Obata K, Takeda K, Shinozaki H (1970) Further study on pharmacological properties of the cerebellar-induced inhibition of Deiters neurones. Exp Brain Res 11: 327–342
Oertel WH, Schmechel DE, Mugnaini E, Tappaz ML, Kopin IJ (1981) Immunocytochemical localization of glutamate decarboxylase in rat cerebellum with a new antiserum. Neuroscience 6: 2715–2735
Okamoto K, Sakai Y (1981) Inhibitory actions of taurocyamine, hypotaurine, homotaurine, taurine and GABA on spike discharges of Purkinje cells, and localization of sensitive sites, in guinea pig cerebellar slices. Brain Res 206: 371–386
Okamoto K, Kimura H, Sakai Y (1983a) Evidence for taurine as an inhibitory neurotransmitter in cerebellar stellate interneurons: selective antagonism by TAG (6-aminomethyl-3methyl-4H,1,2,4-benzothiadiazine-1,1-dioxide). Brain Res 265: 163–168
Okamoto K, Kimura H, Sakai Y (1983b) Antagonistic action of 6aminomethyl-3-methyl-4H,1,2,4-benzothiadiazine-1,1-dioxide (TAG), and evidence for a transmitter role of taurine in stellate interneurons in the cerebellum. In: Kuriyama K, Huxtable R, Iwata H (eds) Sulfur amino acids: biochemical and clinical aspects. Liss, New York, pp 151–160
Okamoto K, Kimura H, Sakai Y (1983c) Taurine-induced increase of the Cl-conductance of cerebellar Purkinje cell dendrites in vitro. Brain Res 259: 319–323
Otsuka M, Obata K, Miyata Y, Tanaka Y (1971) Measurement of γ-aminobutyric acid in isolated nerve cells of cat central nervous system. J Neurochem 18: 287–295
Ottersen OP (1987) Postembedding light and electron microscopic immunocytochemistry of amino acids: description of a new model system allowing identical conditions for specificity testing and tissue processing. Exp Brain Res 69: 167–174
Ottersen OP (1988) Quantitative assessment of taurine-like immunoreactivity in different cell types and processes in rat cerebellum: an electron-microscopic study based on a posternbedding immunogold labelling procedure. Anat Embryol (in press)
Ottersen OP, Storm-Mathisen J (1984) Glutamate and GABAcontaining neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol 229: 374–392
Ottersen OP, Madsen S, Meldrum BS, Storm-Mathisen J (1985) Taurine in the hippocampal formation of the Senegalese baboon Papio papio: an immunocytochemical study with an antiserum against conjugated taurine. Exp Brain Res 59: 457–462
Ottersen OP, Storm-Mathisen J, Madsen S, Skumlien S, Strømhaug J (1986) Evaluation of the immunocytochemical method for amino acids. Med Biol 64: 147–158
Ottersen OP, Storm-Mathisen J, Somogyi P (1988) Colocalization of glycine-like and GABA-like immunoreactivities in Golgi cell terminals in the rat cerebellum: a postembedding light and electron microscopic study. Brain Res (in press)
Ribak CE, Vaughn JE, Saito K (1978) Immunocytochemical localization of glutamic acid decarboxylase in neuronal somata following colchicine inhibition of axonal transport. Brain Res 140: 315–332
Saito K, Barber R, Wu JY, Matsuda T, Roberts E, Vaughn JE (1974) Immunocytochemical localization of glutamate decarboxylase in rat cerebellum. Proc Natl Acad Sci USA 71: 269–273
Schon F, Iversen LL (1972) Selective accumulation of [3H]GABA by stellate cells in rat cerebellar cortex in vivo. Brain Res 42: 503–507
Seguela P, Gamrani H, Geffard M, Calas A, Le Moal M (1985) Ultrastructural immunocytochemistry of γ-aminobutyrate in the cerebral and cerebellar cortex of the rat. Neuroscience 16: 865–874
Somogyi P, Soltész I (1986) Immunogold demonstration of GABA in synaptic terminals of intracellularly recorded, horseradish peroxidase-filled basket cells and clutch cells in the cat's visual cortex. Neuroscience 19: 1051–1065
Somogyi P, Hodgson AJ, Chubb IW, Penke B, Erdei A (1985) Antisera to γ-aminobutyric acid. II. Immunocytochemical application to the central nervous system. J Histochem Cytochem 33: 240–248
Somogyi P, Hodgson AJ, Smith AD, Nunzi MG, Gorio A, Wu J-Y (1984) Different populations of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin or cholecystokinin-immunoreactive material. J Neurosci 4: 2590–2603
Sturman JA, Moretz RC, French JH, Wisniewski HM (1985) Taurine deficiency in the developing cat: persistence of the cerebellar external granule cell layer. J Neurosci Res 13: 405–416
Wu J-Y, Denner LA, Wei SC, Lin C-T, Song G-X, Xu YF, Liu JW, Lin HS (1986) Production and characterization of polyclonal and monoclonal antibodies to rat brain L-glutamate decarboxylase. Brain Res 373: 1–14
Yarbrough GG, Singh DK, Taylor DA (1981) Neuropharmacological characterization of a taurine antagonist. J Pharmacol Exp Ther 219: 604–613
Yoshida M, Karasawa N, Ito M, Sakai M, Nagatsu I (1986) Demonstration of taurine-like immunoreactive structures in the rat brain. Neurosci Res 3: 356–363
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ottersen, O.P., Madsen, S., Storm-Mathisen, J. et al. Immunocytochemical evidence suggests that taurine is colocalized with GABA in the Purkinje cell terminals, but that the stellate cell terminals predominantly contain GABA: a light- and electronmicroscopic study of the rat cerebellum. Exp Brain Res 72, 407–416 (1988). https://doi.org/10.1007/BF00250262
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00250262