Summary
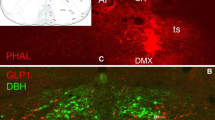
The cellular localization of neurons expressing cholecystokinin (CCK) and tyrosine hydroxylase (TH) mRNAs was analysed in rat ventral mesencephalon using in situ hybridization techniques with both complementary DNA and synthetic oligonucleotide probes. Cell bodies distributed throughout the substantia nigra, ventral tegmental area, interfascicular nucleus, midline raphe nuclei, and central and ventral periaqueductal grey matter were found to contain CCK mRNA or TH mRNA as indicated by high densities of grains overlying the perikarya. The in situ hybridization technique was combined with immunocytochemistry on the same tissue section to localize the peptide or enzyme within its respective mRNA-containing somata. In addition, the presence of TH immunoreactivity was demonstrated within cell bodies labeled for CCK mRNA and immunostaining for CCK was detected within TH mRNA-containing neurons. In the medial geniculate nucleus a strong labeling for CCKmRNA was observed, in spite of the fact that so far no CCK-like immunoreactivity has been demonstrated in perikarya in this nucleus. The specificity of the probes was verified by RNA blot hybridization. These results confirm recent double-labeling immunocytochemical studies and further characterize the coexistence of CCK and TH at the level of their mRNAs as well as their post-translational products in a large population of mesencephalic dopamine neurons known to project to forebrain areas.
Similar content being viewed by others
References
Berod A, Faucon Biguet N, Dumas S, Bloch B, Mallet J (1987) Modulation of tyrosine hydroxylase gene expression in the central nervous system visualized by in situ hybridization. Proc Natl Acad Sci USA 84: 1699–1703
Björklund A, Lindvall O (1984) Dopamine-containing systems in the CNS. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, Vol 2. Classical transmitters in the CNS, Part I. Elsevier, Amsterdam, pp 55–122
Blumstein LK, Crawley JN, Davis LG, Baldino F Jr (1987) Neuropeptide modulation of apomorphine-induced stereotyped behavior. Brain Res 404: 293–300
Bruyn GW (1968) Huntington's chorea: historical, clinical and laboratory synopsis. In: Vinkin PJ, Bruyn GW (eds) Handbook of clinical neurology, Vol 6. Diseases of the basal ganglia. North Holland, Amsterdam, pp 298–378
Bönnemann C, Giraud P, Eiden LE, Meyer DK (1987) Measurement of mRNA specific for preprocholecystokinin in rat caudatoputamen and areas projecting to it. Neurochem Int 10: 521–524
Chesselet M-F, Weiss L, Wuenschell C, Tobin AJ, Affolter HU (1987) Comparative distribution of mRNAs for glutamic acid decarboxylase, tyrosine hydroxylase, and tachykinins in the basal ganglia: an in situ hybridization study in the rodent brain. J Comp Neurol 262: 125–140
Cho HJ, Shiotani Y, Shiosaka S, Inagaki S, Kubota Y, Kiyama H, Umegaki K, Tateishi K, Hashimura E, Hamaoka T, Tohyama M (1983) Ontogeny of cholecystokinin-8-containing neuron system of the rat: an immunohistochemical analysis. I. Forebrain and upper brainstem. J Comp Neurol 218: 25–41
Coons AH (1958) Fluorescent antibody methods. In: Danielli IF (ed) General cytochemical methods. Academic Press, New York, pp 399–422
Crawley JN, Hommer DW, Skirboll LR (1984) Behavioral and neurophysiological evidence for a facilitatory interaction between co-existing transmitters: cholecystokinin and dopamine. Neurochem Int 6: 755–760
Crawley JN, Stivers JA, Blumstein LK, Paul SM (1985) Cholecystokinin potentiates dopamine-mediated behaviors: evidence for modulation specific to a site of coexistence. J Neurosci 5: 1972–1983
Dahlström A, Fuxe K (1964) Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand [Suppl] 62: 1–55
Deschênes RJ, Lorenz LJ, Haun RS, Roos BA, Collier KJ, Dixon JE (1984) Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci USA 81: 726–730
Deschênes RJ, Haun RS, Funckes CL, Dixon JE (1985) A gene encoding rat cholecystokinin: isolation, nucleotide sequence, and promotor activity. J Biol Chem 260: 1280–1286
Duchemin AM, Quach TT, Iadarola MJ, Deschênes RJ, Schwartz JP, Wyatt RJ (1987) Expression of the cholecystokinin gene in rat brain during development. Dev Neurosci 9: 61–67
Fink JS, Smith GP (1980) Mesolimbicocortical dopamine terminal fields are necessary for normal locomotor and investigatory exploration in rats. Brain Res 199: 359–384
Friedman J, Schneider BS, Powell D (1985) Differential expression of the mouse cholecystokinin gene during brain and gut development. Proc Natl Acad Sci USA 82: 5593–5597
Gendelman HE, Moench TR, Narayan O, Griffin DE, Clements JE (1985) A double labeling technique for performing immunocytochemistry and in situ hybridization in virus infected cell cultures and tissues. J Virol Methods 11: 93–103
Gilles C, Lotstra F, Vanderhaeghen J-J (1983) CCK nerve terminals in rat striatum and limbic areas originate partly in the brain stem and partly in telencephalic areas. Life Sci 32: 1683–1690
Grima B, Lamouroux A, Blanet F, Faucon Biguet N, Mallet J (1985) Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci USA 82: 617–621
Gubler U, Chua AO, Hoffman BJ, Collier KI, Eng I (1984) Cloned cDNA to cholecystokinin mRNA predicts an identical preprocholecystokinin in pig brain and gut. Proc Natl Acad Sci USA 81: 4307–4310
Hamilton M, Sheehan MJ, De Belleroche J, Herberg LJ (1986) The cholecystokinin analogue, caerulein, does not modulate dopamine release or dopamine-induced locomotor activity in the nucleus accumbens of rat. Neurosci Lett 44: 77–82
Han VKM, Snouweart J, Towle AC, Lund PK, Lauder JM (1987) Cellular localization of tyrosine hydroxylase mRNA and its regulation in the rat adrenal medulla and brain by in situ hybridization with an oligodeoxyribonucleotide probe. J Neurosci Res 17: 11–18
Harlan RE, Shivers BD, Romano GJ, Howells RD, Pfaff DW (1987) Localization of preproenkephalin mRNA in the rat brain and spinal cord by in situ hybridization. J Comp Neurol 258: 159–184
Hasegawa M, Usui H, Araki K, Kuwano R, Takahashi Y (1986) Developmental and regional changes of cholecystokinin mRNA in rat brain. FEBS Lett 194: 224–226
Hökfelt T, Skirboll L, Rehfeld JF, Goldstein M, Markey K, Dann O (1980) A subpopulation of mesencephalic dopamine neurons projecting to limbic areas contains a cholecystokininlike peptide: evidence from immunohistochemistry combined with retrograde tracing. Neuroscience 5: 2093–2124
Hökfelt T, Mårtensson R, Björklund A, Kleinau S, Goldstein M (1984a) Distributional maps of tyrosine hydroxylase-immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, Vol 2. Classical transmitters in the CNS, Part I. Elsevier, Amsterdam, pp 277–379
Hökfelt T, Johansson O, Goldstein M (1984b) Central catecholamine neurons as revealed by immunohistochemistry with special reference to adrenaline neurons. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, Vol 2. Classical transmitters in the CNS, Part I. Elsevier, Amsterdam, pp 157–276
Hökfelt T, Holets VR, Staines W, Meister B, Melander T, Schalling M, Schultzberg M, Freedman J, Björklund H, Olson L, Lindh B, Elfrin L-G, Lundberg JM, Lindgren JÅ, Samuelsson B, Pernow B, Terenius L, Post C, Everitt B, Goldstein M (1986) Coexistence of neuronal messengers — an overview. Prog Brain Res 68: 33–70
Hökfelt T, Herrera-Marschitz M, Seroogy K, Ju G, Holets V, Schalling M, Ungerstedt U, Post C, Rehfeld JF, Frey P, Fischer J, Dockray G, Hamaoka T, Walsh JH, Goldstein M (1988) Immunohistochemical studies on cholecystokinin (CCK)-immunoreactive neurons in the rat using sequencespecific antisera and with special reference to the caudate nucleus and primary sensory neurons. J Chem Neuroanat 1: 11–52
Hornykiewicz O (1980) Biochemical abnormalities in some extrastriatal neuronal systems in Parkinson's disease. In: Rinne UK, Klinger M, Stan G (eds) Parkinson's disease, current progress, problems and management. Elsevier, Amsterdam, pp 109–119
Hunt CA, Seroogy KB, Gall CM, Jones EG (1987) Cholecystokinin innervation of rat thalamus, including fibers to ventroposterolateral nucleus from dorsal column nuclei. Mol Brain Res 426: 257–269
Innis RB, Correa FM, Uhl GR, Schneider B, Snyder SH (1979) Cholecystokinin octapeptide-like immunoreactivity: histochemical localization in rat brain. Proc Natl Acad Sci USA 76: 521–525
Javoy-Agid F, Ruberg M, Tequet H, Bokobza B, Agid Y, Gaspar P, Breger B, N'Guyen-Legros J, Alvarez C, Gray F, Escourolle R, Scatton B, Rouquier L (1984) Biochemical neuropathology of Parkinson's disease. Adv Neurol 40: 189–198
Johnson DG, de C Nogueira Araujo GM (1981) A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods 43: 349–350
Ju G, Hökfelt T, Fischer JA, Frey P, Rehfeld JF, Dockray GJ (1986) Does cholecystokinin-like immunoreactivity in rat primary sensory neurons represent calcitonin gene-related peptide? Neurosci Lett 68: 305–310
Ju G, Hökfelt T, Brodin E, Fahrenkrug J, Fischer JA, Frey P, Elde RP, Brown JC (1987) Primary sensory neurons of the rat showing calcitonin gene-related peptide (CGRP) immunoreactivity and their relation to substance P-, somatostatin-, galanin-, vasoactive intestinal polypeptideand cholecystokinin-immunoreactive ganglion cells. Cell Tissue Res 247: 417–431
Kuwano R, Araki K, Usui H, Fukui T, Ohtsuka E, Ikehara M, Takahashi Y (1984) Molecular cloning and nucleotide sequence of cDNA coding for rat brain cholecystokinin precursor. J Biochem 96: 923–926
Lamouroux A, Faucon Biguet N, Samolyk D, Privat A, Salomon JC, Pujol JF, Mallet J (1982) Identification of cDNA clones coding for rat tyrosine hydroxylase antigen. Proc Natl Acad Sci USA 79: 3881–3885
Lindvall O, Björklund A (1983) Dopamine- and norepinephrine-containing neuron systems: their anatomy in the rat brain. In: Emson PC (ed) Chemical neuroanatomy. Raven Press, New York, pp 229–255
Lorén I, Alumets J, Håkanson R, Sundler F (1979) Distribution of gastrin and CCK-like peptides in rat brain: an immunocytochemical study. Histochemistry 59: 249–257
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Markey KA, Kondo S, Shenkman L, Goldstein M (1980) Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol 17: 79–85
Markstein R, Hökfelt T (1984) Effect of cholecystokinin-octapeptide on dopamine release from slices of cat caudate nucleus. J Neurosci 4: 570–575
Marley PD, Emson PC, Rehfeld JH (1982) Effect of 6-hydroxydopamine lesions of the medial forebrain bundle on the distribution of cholecystokinin in rat forebrain. Brain Res 252: 382–385
Mason ST (1984) Catecholamines and behavior. Cambridge University Press, Cambridge
Matthysse SW, Kety SS (1975) Catecholamines and schizophrenia. Pergamon Press, Oxford
Meltzer HY, Stahl SM (1976) The dopamine hypothesis of schizophrenia: a review. Schizophr Bull 2: 19–76
Mutt V, Jorpes JE (1966) Isolation of aspartyl-phenylalanine amide from cholecystokinin-pancreozymin. Biochem Biophys Res Commun 26: 392–397
Mutt V, Jorpes JE (1968) Structure of porcine cholecystokinin-pancreozymin. I. Cleavage with thrombin and with trypsin. Eur J Biochem 6: 156–162
Nair NPV, Lal S, Bloom DM (1986) Cholecystokinin and schizophrenia. Prog Brain Res 65: 237–258
Pease PC (1962) Buffered formaldehyde as a killing agent and primary fixative for electron microscopy. Anat Rec 142: 342
Platt JL, Michael AF (1983) Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem 31: 840–842
Randrup A, Munkvad I (1972) Evidence indicating an association between schizophrenia and dopaminergic hyperactivity in the brain. Orthomol Psychiatr 1: 2–7
Rehfeld JF (1987) Preprocholecystokinin processing in the normal human anterior pituitary. Proc Natl Acad Sci USA 84: 3019–3023
Réthelyi M, McGehee D, Lund PK (1986) Neuronal localization of cholecystokinin mRNAs in rat and guinea pig. Soc Neurosci Abstr 12: 1041
Roberts GW, Ferrier IN, Lee Y, Crow TJ, Johnstone EC, Owens DGC, Bacarese-Hamilton AJ, McGregor G, O'Shaughnessy D, Polak JM, Bloom SR (1983) Peptides, the limbic lobe and schizophrenia. Brain Res 288: 199–211
Schalling M, Hökfelt T, Wallace B, Goldstein M, Filer D, Yamin C, Schlessinger DH (1986) Tyrosine 3-hydroxylase in rat brain and adrenal medulla: hybridization histochemistry and immunohistochemistry combined with retrograde tracing. Proc Natl Acad Sci USA 83: 6208–6212
Schalling M, Dagerlind Å, Brené S, Petterson R, Carrol JM, Joh TH, Hökfelt T (1987) Localization of mRNA for phenylethanolamine-N-methyltransferase (PNMT) using in situ hybridization. Acta Physiol Scand 131: 631–632
Schalling M, Seroogy K, Hökfelt T, Chai SY, Hallman H, Persson H, Larhammar D, Ericsson A, Terenius L, Graffi J, Massoulié J, Goldstein M (1988) Neuropeptide Y in the rat adrenal gland — immunohistochemical and in situ hybridization studies. Neuroscience 24: 337–349
Schneider LH, Albert JE, Iversen SD (1983) CCK-8 modulation of mesolimbic dopamine: antagonism of amphetamine-stimulated behaviors. Peptides 4: 749–753
Segerson TP, Hoefler H, Childers H, Wolfe HJ, Wu P, Jackson IMD, Lechan RM (1987) Localization of thyrotropin-releasing hormone prohormone messenger ribonucleic acid in rat brain by in situ hybridization. Endocrinology 121: 98–107
Seroogy KB (1986) Cholecystokinin innervation of rat forebrain. Ph. D. Dissertation, University of California, Irvine
Seroogy KB, Fallon JH (1988) Forebrain projections from cholecystokinin-like immunoreactive neurons in rat midbrain. J Comp Neurol (in press)
Seroogy KB, Mehta A, Fallon JH (1987a) Neurotensin and cholecystokinin coexistence within neurons of the ventral mesencephalon: projections to forebrain. Exp Brain Res 68: 277–289
Seroogy K, Schalling M, Chai SY, Hökfelt T, Persson H, Dixon J, Filer D, Goldstein M (1987b) Dopamine/CCK coexistence in rat ventral mesencephalon as determined by in situ hybridization histochemistry combined with immunocytochemistry. Neuroscience [Suppl] 22: S305
Seroogy K, Schalling M, Brené S, Dagerlind Å, Chai SY, Persson H, Dixon J, Filer D, Goldstein M, Walsh J, Hökfelt T (1987c) Combined in situ hybridization and immunohistochemistry for CCK and tyrosine hydroxylase in rat ventral midbrain. Soc Neurosci Abstr 13: 1088
Seroogy KB, Dangaran K, Lim S, Haycock J, Fallon JH (1988) Ventral mesencephalic neurons containing both cholecystokininand tyrosine hydroxylase-like immunoreactivities project to forebrain regions. J Comp Neurol (in press)
Shivers BD, Harlan RE, Pfaff DW, Schachter BS (1986) Combination of immunocytochemistry and in situ hybridization in the same tissue section of rat pituitary. J Histochem Cytochem 34: 39–43
Siegel RE, Young WS III (1985) Detection of preprocholecystokinin and preproenkephalin A mRNAs in rat brain by hybridization histochemistry using complementary RNA probes. Neuropeptides 6: 573–580
Siegel RE, Young WS III (1986) Detection of neuropeptide mRNAs by in situ hybridization histochemistry. In: Uhl GR (ed) In situ hybridization in brain. Plenum Press, New York, pp 63–71
Skirboll LR, Grace AA, Hommer DW, Rehfeld J, Goldstein M, Hökfelt T, Bunney BS (1981) Peptide-monoamine coexistence: actions of cholecystokinin-like peptide on the electrical activity of midbrain dopamine neurons. Neuroscience 11: 2111–2124
Snyder SH, Banerju SP, Yamamura HI, Greenberg D (1974) Drugs, neurotransmitters and schizophrenia. Science 184: 1243–1253
Spokes EGS (1980) Neurochemical alterations in Huntington's chorea: a study of post-mortem brain tissue. Brain 103: 179–210
Studler JM, Simon H, Cesselin F, Legrand JC, Glowinski J, Tassin JP (1981) Biochemical investigation on the cholecystokinin octapeptide in dopaminergic neurons originating from the ventral tegmental area of the rat. Neuropeptides 2: 131–139
Studler JM, Javoy-Agid F, Cesselin F, Legrand JC, Agid Y (1982) CCK-8-immunoreactivity distribution in human brain: selective decrease in the substantia nigra from parkinsonian patients. Brain Res 243: 176–179
Studler JM, Reibaud M, Tramu G, Blanc G, Glowinski J, Tassin JP (1984) Pharmacological study on the mixed CCK8/DA meso-nucleus accumbens pathway: evidence for the existence of storage sites containing the two transmitters. Brain Res 298: 91–97
Studler JM, Javoy-Agid F (1985) Cholecystokinin octapeptide immunoreactivity in human brain: modifications in parkinsonian patients. Ann NY Acad Sci 448: 656–659
Takahashi Y, Kato K, Hayashizaki Y, Wakabayashi T, Ohtsuka E, Atsuki S, Ikehara M, Matsubara K (1985) Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci USA 82: 1931–1935
Vanderhaeghen J-J, Lotstra F, De Mey J, Gilles C (1980) Immunochemical localization of cholecystokinin- and gastrin-like peptides in the brain and hypophysis of the rat. Proc Natl Acad Sci USA 77: 1190–1194
Verbanck PMP, Lotstra F, Gilles C, Linkowski P, Mendelwicz J, Vanderhaeghen J-J (1984) Reduced cholecystokinin immunoreactivity in the cerebrospinal fluid of patients with psychiatric disorders. Life Sci 34: 67–72
Wang RY, Hu X-T (1986) Does cholecystokinin potentiate dopamine action in the nucleus accumbens? Brain Res 380: 363–367
Wang RY, White FJ, Voigt MM (1984) Cholecystokinin, dopamine, and schizophrenia. Trends Pharmacol 5: 436–438
Wang RY, White FJ, Voigt MM (1985) Interactions of cholecystokinin and dopamine in the nucleus accumbens. Ann NY Acad Sci 448: 352–360
White FJ, Wang RY (1984) Interactions of cholecystokinin octapeptide and dopamine on nucleus accumbens neurons. Brain Res 300: 161–166
Whittemore SR, Ebendal T, Lärkfors L, Olson L, Seiger Å, Stromberg I, Persson H (1986) Developmental and regional expression of β-nerve growth factor messenger RNA and protein in the rat central nervous system. Proc. Natl. Acad. USA 83: 817–821
Widerlöv E, Kalivas PW, Lewis MH, Prange AJ Jr, Breese GR (1983) Influence of cholecystokinin on central monoaminergic pathways. Regul Pept 6: 99–109
Williams RG, Gayton RJ, Zhu W-Y, Dockray GJ (1981) Changes in brain cholecystokinin octapeptide following lesions of the medial forebrain bundle. Brain Res 213: 227–230
Wolfson B, Manning RW, Davis LG, Arentzen R, Baldino F Jr (1985) Colocalization of corticotropin releasing factor and vasopressin mRNA in neurons after adrenalectomy. Nature 315: 59–61
Young WS III, Bonner TI, Brann MR (1986a) Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci USA 83: 9827–9831
Young WS III, Mezey E, Siegel RE (1986b) Vasopressin and oxytocin mRNAs in adrenalectomized and Brattleboro rats: analysis by quantitative in situ hybridization histochemistry. Mol Brain Res 1: 231–241
Young WS III, Mezey E, Siegel RE (1986c) Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett 70: 198–203
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seroogy, K., Schalling, M., Brené, S. et al. Cholecystokinin and tyrosine hydroxylase messenger RNAs in neurons of rat mesencephalon: peptide/monoamine coexistence studies using in situ hybridization combined with immunocytochemistry. Exp Brain Res 74, 149–162 (1989). https://doi.org/10.1007/BF00248288
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248288