Abstract
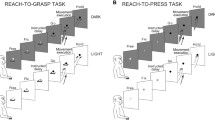
Movement extent and movement force can be independently controlled in motor performance. Therefore, independent representations of extent and force should exist in the central nervous system (CNS). To test this hypothesis, microelectrode recordings were made in sensorimotor cortex of monkeys trained to perform visually cued wrist flexion movements of two extents, against two levels of frictional resistance. An initial preparatory signal (PS) provided complete, partial or no information about extent and/or force of the movement, which had to be performed in response to a second, response signal (RS). The activity of 511 neurons of the primary motor cortex (MI), the premotor cortex (PM), the postcentral cortex (PC), and the posterior parietal cortex (PA) was recorded in two monkeys. Both reaction time (RT) and neuronal data suggest that there exist independent, neuronal mechanisms responsible for the programming of either parameter. On the one hand, partial information about either movement parameter shortened RT when compared with the condition of no prior information. On the other hand, there were, among others, two discrete populations of neurons, one related only to extent, the other only to force. Preparatory changes in activity related to either movement parameter were mainly located in the frontal cortex, especially in the PM. After occurrence of the RS, the percentage of selective changes in activity increased and tended to extend to the parietal cortex. In particular during the movement, force-related changes in activity have been encountered in PA. Furthermore, we conducted trial-by-trial correlation analyses between RT and preparatory neuronal activity for all conditions of prior information. The mean correlation coefficient was significantly higher in the condition of information about movement extent than of information about movement force and it was significantly higher in MI/ PM than in PC/PA.
Similar content being viewed by others
References
Alexander GE (1987) Selective neuronal discharge in monkey putamen reflects intended direction of planned limb movements. Exp Brain Res 67:623–634
Andersen RA, Asanuma C, Essick G, Siegel RM (1990) Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol 296:65–113
Bioulac R, Lamarre Y (1979) Activity of postcentral cortical neurons of the monkey during conditioned movements of a deafferented limb. Brain Res 172:427–437
Blatt GJ, Andersen RA, Stoner GR (1990) Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol 299:421–445
Bon L, Lucchetti C (1992) The dorsomedial frontal cortex of the macaque monkey: fixation- and saccade-related activity. Exp Brain Res 89:571–580
Bonnet M, Requin J, Stelmach GE (1982) Specification of direction and extent in motor programming. Bull Psychon Soc 19:31–34
Bonnet M, Requin J, Stelmach GE (1991) Changes in electromyographical responses to muscle stretch, related to the programming of movement parameters. Electroencephalogr Clin Neurophysiol 81:135–151
Crammond DJ, Kalaska JF (1989) Neuronal activity in primate parietal cortex area 5 varies with intended movement direction during an instructed-delay period. Exp Brain Res 76:458–462
Evarts EV (1981) Role of motor cortex in voluntary movements in primates. In: Brooks VB (eds) Motor control. (Handbook of physiology, sect 1, The nervous system, vol II) American Physiological Society, Bethesda, pp 1083–1120
Evarts EV, Shinoda Y, Wise SP (1984) Neurophysiological approaches to higher brain functions. Wiley, New York
Fromm C, Evarts EV (1982) Pyramidal tract neurons in somatosensory cortex: central and peripheral inputs during voluntary movement. Brain Res 238:186–191
Georgopoulos AP, Ashe J, Smyrnis N, Taira M (1992) The motor cortex and the coding of force. Science 256:1692–1695
Ghez C, Hening W, Favilla M (1990) Parallel interacting channels in the initiation and specification of motor responses. In: Jeannerod M (eds) Attention and performance, vol XIII. Erlbaum, Hillsdale, NJ, pp 265–293
Godschalk M, Lemon RN (1989) Preparation of visually cued arm movements in monkey. Involvement of inferior parietal cortex. Brain Behav Evol 33:122–126
He S-Q, Dum RP, Strick PL (1993) Topographical organization of corticospinal projections from the spinal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci 13:952–980
Hepp-Reymond MC (1988) Functional organization of motor cortex and its participation in voluntary movements. In: Steklis HD, Erwin J (eds) Neurosciences. (Comparative primate biology, vol 4) Liss, New York, pp 501–624
Humphrey DR, Tanji J (1991) What features of voluntary motor control are encoded in the neuronal discharge of different cortical motor areas? In: Humphrey DR, Freund H-J (eds) Motor control: concepts and issues. Wiley, Chichester, pp 413–443
Kalaska JF, Hyde ML (1985) Area 4 and area 5: differences between the load direction-dependent discharge variability of cells during active postural fixation. Exp Brain Res 59:197–202
Kalaska JF, Cohen DA, Prud'homme M, Hyde ML (1990) Parietal area 5 neuronal activity encodes movement kinematics, not movement dynamics. Exp Brain Res 80:351–364
Kubota K, Funahashi S (1982) Direction-specific activities of dorsolateral prefrontal and motor cortex pyramidal tract neurons during visual tracking. J Neurophysiol 47:362–376
Kurata K (1993) Premotor cortex of monkeys: set- and movement-related activity reflecting amplitude and direction of wrist movements. J Neurophysiol 69:187–200
Larish DD, Frekany GA (1985) Planning and preparing expected and unexpected movements: reexamining the relationships of arm, direction, and extent of movement. J Mot Behav 17:168–189
Lépine D, Glencross D, Requin J (1989) Some experimental evidence for and against a parametric conception of movement programming. J Exp Psychol [Hum Percept] 15:347–362
MacKay WA (1992) Properties of reach-related neuronal activity in cortical area 7a. J Neurophysiol 67:1335–1345
MacKay WA, Bonnet M (1990) CNV, stretch reflex and reaction time correlates of preparation for movement direction and force. Electroencephalogr Clin Neurophysiol 76:47–62
Mackay WA, Riehle A (1991) Correlates of preparation of arm reach parameters in parietal area 7a of the cerebral cortex. In: Requin J, Stelmach GE (eds) Tutorials in motor neuroscience. Kluwer, Dordrecht, pp 347–356
MacKay WA, Riehle A (1992) Planning a reach: spatial analysis by area 7a neurons. In: Stelmach GE, Requin J (eds) Tutorials in motor behavior, vol II. Elsevier, Amsterdam, pp 501–514
Matelli M, Lupino G, Rizzolatti G (1985) Patterns of cytochrome oxidase activity in the frontal agranular cortex of macaque. Behav Brain Res 18:125–137
Niki H (1974) Differential activity of prefrontal units during right and left delayed response trials. Brain Res 70:346–349
Powell TPS, Mountcastle VB (1959) The cytoarchitecture of the postcentral gyrus of the monkey Macaca mulatta. Bull Johns Hopkins Hosp 105:108–131
Prochazka A (1989) Sensorimotor gain control — a basic strategy of motor systems. Progr Neurobiol 33:281–307
Requin J, Riehle A, Seal J (1988) Neuronal activity and information processing in motor control: from stages to continuous flow. Biol Psychol 26:179–198
Requin J, Brener J, Ring C (1991) Preparation for action. In: Jennings RR, Coles MG H (eds) Handbook of cognitive psychophysiology: central and autonomic nervous system approaches. Wiley, New York, pp 357–448
Requin J, Riehle A, Seal J (1993) Neuronal networks for movement preparation. In: Meyer DE, Kornblum S (eds) Attention and performance, vol XIV. MIT, Cambridge, MA, pp 745–769
Riehle A, MacKay WA (1992) Are force and extent independent movement parameters? Soc Neurosci Abstr 18:502
Riehle A, Requin J (1989) Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. J Neurophysiol 61:534–549
Riehle A, Requin J (1993) The predictive value for performance speed of preparatory changes in activity of the monkey motor and premotor cortex. Behav Brain Res 53:35–49
Rosenbaum DA (1980) Human movement initiation: specification of arm, direction, and extent. J Exp Psychol [Gen] 109:444–474
Rosenbaum DA (1983) The movement precuing technique: assumptions, applications, and extensions. In: Magill RA (ed) Memory and control of action. North-Holland, Amsterdam, pp 231–274
Sessle BJ, Wiesendanger M (1982) Structural and functional definition of the motor cortex in the monkey (Macaca fascicularis). J Physiol (Lond) 323:245–265
Strick PL (1983) The influence of motor preparation on the response of cerebellar neurons to limb displacements. J Neurophysiol 3:2007–2020
Tanji J, Evarts EV (1976) Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol 39:1062–1068
Vidal F, Bonnet M, Macar F (1991) Programming response duration in a precueing reaction time paradigm. J Mot Behav 23:226–234
Wannier TMJ, Töltl M, Hepp-Reymond MC (1986) Neuronal activity in the postcentral cortex related to force regulation during a precision grip task. Brain Res 382:427–432
Weinrich M, Wise SP (1982) The premotor cortex of the monkey. J Neurosci 2:1329–1345
Wise SP (1984) The nonprimary motor cortex and its role in the cerebral control of movement. In: Edelman G, Gall WE, Cowan WH (eds) Dynamic aspects of neocortical function. Wiley, New York, pp 525–555
Wise SP (1989) Frontal cortex activity and motor set. In: Ito M (ed) Neural programming. (Taniguchi symposia on the brain sciences, No. 12) Karger, Basel, pp 25–38
Zelaznik HN, Hahn R (1985) Reaction time methods in the study of motor programming: the precuing of hand, digit, and duration. J Mot Behav 17:190–218
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Riehle, A., MacKay, W.A. & Requin, J. Are extent and force independent movement parameters? Preparation- and movement-related neuronal activity in the monkey cortex. Exp Brain Res 99, 56–74 (1994). https://doi.org/10.1007/BF00241412
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00241412