Summary
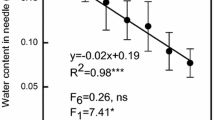
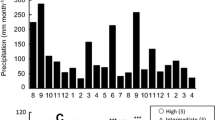
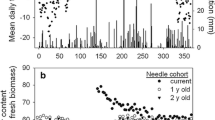
A transient decline in photosynthetic rate and several correlates of photosynthetic function in year-old shade needles coincided with shoot elongation in 15 fullsib 8-year-old Douglas-fir [Pseudotsuga menziesii (Mirb.) Franco] saplings. In year-old needles and current year needles collected from May to November from branchlets with a single terminal bud and at least 4 needle age classes, chlorophyll (chl) content, photosynthetic rate and non-photochemical quenching of chl fluorescence declined during the period of flushing of the new shoots and recovered as shoot elongation slowed. Developing shade needles did not achieve the same oxygen evolution rate per unit area as the year-old needles, but did develop a higher quantum yield (estimated from chl a fluorescence). In short, in shade branchlets shoot development occurred at a cost of photosynthetic function in year-old needles. In year-old sun needles collected from the upper portions of the same trees, total protein concentration increased prior to, and decreased during, flushing. The concentration of ribulose 1,5-bisphosphate carboxylase-oxygenase (Rubisco) rose and decreased more than chlorophyll-binding proteins. In general, protein concentration in needles reflected age class rather than sun or shade environment. A specific decline in Rubisco in year-old sun needles during the period of new shoot elongation strengthens the hypothesis that degradation of this photosynthetic protein contributes to development of the new shoot.
Similar content being viewed by others
References
Camm EL, Green BR, Allred DR, Staehelin LA (1987) Association of the 33 kDa extrinsic polypeptide (water-splitting) with PSII particles; immunochemical quantification of residual polypeptide after membrane extraction. Photosynthesis Res 13: 69–80
Demmig B, Bjorkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 184: 538–544
Dietz K-J, Schreiber U, Heber U (1985) The relationship between the redox state of Qa and photosynthesis in leaves at various carbondioxide and light regimes. Planta 166: 219–226
van den Driessche R (1984) Nutrient storage, retranslocation and relationship of stress to nutrition. In Bowen GD, Nambiar EKS (eds) Nutrition of plantation forests. Academic Press, San Diego, pp 181–209
van den Driessche R (1985) Late-season fertilization, mineral nutrient reserves and retranslocation in planted Douglas-fir [Pseudotsuga menziesii (Mirb.) Franco] seedlings. For Sci 31: 485–496
van den Driessche R, Webber JR (1977) Seasonal variation in a Douglas fir stand in total and soluble nitrogen in inner bark and root and in total and mineralizable nitrogen in soil. Can J For Res 7: 641–647
Elrifi IR, Holmes JJ, Weger HP, Mayo WP, Turpin DH (1988) RuBP limitation of photosynthetic carbon fixation during NH3 assimilation: interaction between photosynthesis, respiration and ammonium assimilation in N-limited green algae. Plant Physiol 87: 395–401
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92
Gezelius K, Hallen M (1980) Seasonal variation in ribulose bisphosphate carboxylase activity in Pinus sylvestris. Physiol Plant 48: 88–98
Gifford RM, Evans LT (1981) Photosynthesis, carbon partitioning and yield. Annu Rev Plant Physiol 32: 485–509
Inskeep WP, Bloom PR (1985) Extinction co-efficients of chlorophyll a and b in N, N dimethyl formamide and 80% acetone. Plant Physiol 77: 483–485
Kozlowski TT, Kramer PJ, Pallardy SG (1991) The physiological ecology of woody plants. Academic Press, San Diego, pp 40–43
Krause GH (1988) Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol Plant 74: 566–574
Kreuger KW (1967) Nitrogen, phosphorous and carbohydrates in expanding year-old Douglas-fir shoots. For Sci 13: 352–356
Mehta RA, Fawcett TW, Porath D, Mattoo AK (1992) Oxidative stress causes rapid membrane translocation and in vivo degradation of ribulose-1,5-bisphosphate carboxylase/oxygenase. J Biol Chem 267: 2810–2816
Meyer MM Jr, Splittstoesser WE (1971) The utilization of carbohydrate and nitrogen reserves by Taxus during its spring growth period. Physiol Plant 24: 306–314
Millard P, Proe MF (1992) Storage and internal cycling of nitrogen in relation to seasonal growth of Sitka spruce. Tree Physiol 10: 33–43
Nambiar EKS, Fife DN (1991) Nutrient translocation in temperate conifers. Tree Physiol 9: 185–207
Peoples M, Gifford R (1990) Long distance transport of carbon and nitrogen from sources to sinks in higher plants. In: Dennis DT, Turpin DH (eds) Plant physiology, biochemistry and molecular biology. Longman, UK, pp 442–455
Quick WP, Stitt M (1989) An examination of factors contributing to non-photochemical quenching by chlorophyll fluorescence in barley leaves. Biochim Biophys Acta 977: 287–296
Roberts D, Toivonen P, McInnis SM (1991) Discrete proteins associated with overwintering of interior spruce and Douglas-fir seedlings. Can J Bot 69: 437–441
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynthesis Res 10: 51–62
Turgeon R (1989) The sink source transition in leaves. Annu Rev Plant Physiol Plant Mol Biol 40: 119–38
Troeng E, Linder S (1982) Gas exchange in a 20-year-old stand of Scots pine. 1. Net photosynthesis of current and one-year-old shoots within and between seasons. Physiol Plant 54: 7–14
Vance NC, Copes DO, Zaerr JB (1990) Differences in proteins synthesized in needles of shaded and unshaded Pinus ponderosa var. scopolorum seedlings during prolonged drought. Plant Physiol 92: 1244–1248
Vapaavuori EM, Rikala R, Ryyppb A (1992) Effects of root temperature on growth and photosynthesis in conifer seedlings during shoot elongation. Tree Physiol 10: 217–230
Walker D (1987) The use of the oxygen electrode and fluorescence probes in simple measurements of photosynthesis. Oxygraphics, Sheffield, UK
Webb WL (1975) Dynamics of photoassimilated carbon in Douglas fir seedlings. Plant Physiol 56: 455–459
Wetzel S, Demmers C, Greenwood JS (1991) Protein-storing vacuoles in inner bark and leaves of softwoods. Trees 5: 196–202
Wilkinson L (1989) SYSTAT: the System for Statistics. Systat, Evanston, Ill, pp 450–459
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Camm, E. Photosynthetic responses in developing and year-old Douglas-fir needles during new shoot development. Trees 8, 61–66 (1993). https://doi.org/10.1007/BF00240983
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00240983