Summary
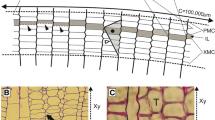
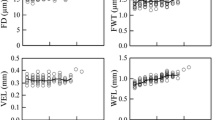
Microscopic examination of the vascular cambium of Eucalyptus globulus, from four trees, at nine height levels, revealed a non-storied cambial structure with both ray and fusiform initials. Average fusiform initial length increased from 443 μm at 70% of tree height from the base to a maximum of 484 μm at 10% of tree height. At 2.5% of tree height average fusiform initial length sharply declined to 438 μm. A strong positive correlation (r = 0.757, df = 7, P <0.05) existed between average fusiform initial length and average fibre length at each height, giving quantitative support to the notion that variation in fusiform initial length is an important mechanism influencing fibre length in hardwoods. However, 62% of the longitudinal variation in average fibre length was explained by the extent of elongation during differentiation, which increased linearly down the stem. At 70% of tree height, the average length of fibres was 1.84 times that of the fusiform initials, compared to 2.11 times at 2.5% of tree height. Concomitant with this longitudinal gradient of elongation was a gradient of increasing tangential area of the cambium occupied by cambial rays. This was accounted for by dimensional increases in the ray initial cells with decreasing height. Possible mechanisms that regulate the elongation of developing fibres and the proportion of cambial rays are discussed.
Similar content being viewed by others
References
Ajmal S, Khan R, Iqbal M (1986) Cambial structure of Holoptelea integrifolia Planch. in relation to age. Flora 178: 197–202
Aloni R (1979) Role of auxin and gibberellin in differentiation of primary phloem fibres. Plant Physiol 63: 609–614
Aloni R (1987) Differentiation of vascular tissues. Annu Rev Plant Physiol 38: 179–204
Aloni R (1988) Vascular differentiation within the plant. In: Timell TE (ed) Vascular differentiation and plant growth regulators. (Springer series in wood science) Springer, Berlin Heidelberg New York, pp 39–62
Aloni R (1992) The control of vascular differentiation. Int J Plant Sci 153: S90-S92
Aloni R, Zimmermann MH (1983) The control of vessel size and density along the plant axis: a new hypothesis. Differentiation 24: 203–208
Bailey IW (1923) The cambium and its derivative tissues. IV. The increase in girth of the cambium. Am J Bot 10: 499–509
Barghoorn ES (1940) The ontogenetic development and phylogenetic specialisation of rays in the xylem of dicotyledons. I. The primitive ray structure. Am J Bot 27: 918–928
Barghoorn ES (1941a) The ontogenetic development and phylogenetic specialisation of rays in the xylem of dicotyledons. II. Modification of the multiseriate and uniseriate rays. Am J Bot 28: 273–282
Barghoorn ES (1941b) The ontogenetic development and phylogenetic specialisation of rays in the xylem of dicotyledons. III. The elimination of rays. Bull Torrey Bot Club 68: 317–325
Denne MP, Dodd RS (1981) The environmental control of xylem differentiation. In: Barnett JR (ed) Xylem cell development. Castle House, Kent, pp 236–255
Digby J, Wareing PF (1966) The effect of applied growth hormones on cambial division and the differentiation of the cambial derivatives. Ann Bot 30: 539–548
Ghouse AKM, Hashmi S (1980) Changes in the vascular cambium of Polyalthia longifolia Benth. et Hook. (Annonaceae) in relation to the girth of the tree. Flora 170: 135–143
Ghouse AKM, Iqbal M (1977) Variation trends in the cambial structure of Prosopis spicigera L. in relation to the girth of the tree axis. Bull Torrey Bot Club 104: 197–201
Hejnowicz A, Hejnowicz Z (1958) Variations of length of vessel members and fibres in the trunk of Populus tremula L. Acta Soc Bot Pol 27: 131–159
Iqbal M, Ghouse AKM (1987) Anatomy of the vascular cambium of Acacia nilotica (L.) Del. var. telia Troup (Mimosaceae) in relation to age and season. Bot J Linn Soc 94: 385–397
Iqbal M, Ghouse AKM (1990) Cambial concept and organisation. In: Iqbal M (ed) The vascular cambium. Research Studies Press, Somerset, pp 1–36
Khan MIH, Khan S, Khan AH, Iqbal S (1979) A comparative study on the variation in cell size of wood fibres and their mother initials in some species of Eucalyptus. Aust For Res 9: 143–145
Khan KK, Ahmad Z, Iqbal M (1981) Trends of ontogenetic size variation of cambial initials and their derivatives in the stem of Bauhinia parviflora Vahl. Bull Soc Bot Fr 128: 165–175
Khan MIH, Siddiqi TO, Khan AH (1983) Ontogenetic changes in the cambial structure of Citrus sinensis L. Flora 173: 151–158
Lev-Yadun S, Aloni R (1991a) Natural and experimentally induced dispersion of aggregate rays in shoots of Quercus ithaburensis Decne. and Q. calliprinos Webb. Ann Bot 68: 85–91
Lev-Yadun S, Aloni R (1991b) Polycentric vascular rays in Suaeda monoica and the control of ray initiation and spacing. Trees 5: 22–29
Lev-Yadun S, Aloni R (1992) The role of wounding and partial girdling in differentiation of vascular rays. Int J Plant Sci 153: 348–357
O'Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Philipson WR, Ward JM, Butterfield BG (1971) The vascular cambium: its development and activity. Chapman & Hall, London
Rao KS, Dave YS (1981) Seasonal variations in the cambial anatomy of Tectona grandis (Verbenaceae). Nord J Bot 1: 535–542
Whetten R, Sederoff R (1991) Genetic engineering of wood. For Ecol Manage 43: 301–316
Zar JH (1984) Biostatistical analysis. Prentice-Hall, London
Zobel BJ, van Buijtenen JP (1989) Wood variation: its causes and control. Springer, Berlin Heidelberg New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ridoutt, B.G., Sands, R. Within-tree variation in cambial anatomy and xylem cell differentiation in Eucalyptus globulus . Trees 8, 18–22 (1993). https://doi.org/10.1007/BF00240977
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00240977