Summary
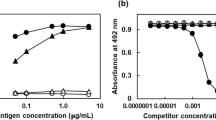
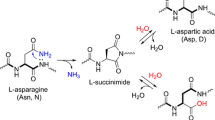
Detection of ε(γ-glutamyl) lysine crosslinks is not only necessary for establishing the importance of the dipeptide as a post-translational modification of proteins, but provides information as to the importance of the transglutaminase enzyme in a biological system. The crosslink may be detected using both indirect and direct methodology. Indirect methods for its detection include measurement of ‘masked lysines’ within a protein, detection of polymer formation by gel-electrophoresis and the inhibition of crosslinking by the incorporation of small molecular weight amines into the substrate protein. Direct methods for the detection of ε(γ-glutamyl) lysine require the actual isolation of the dipeptide following its release from the sample protein by exhaustive proteolytic digestion. Separation of the dipeptide from other components of the digest may be achieved by either ion-exchange chromatography or gel filtration and its qualitative identification achieved by techniques such as paper-electrophoresis or thin layer chromatography. Quantitative estimation of ε(γ-glutamyl) lysine normally involves its further separation by ion-exchange chromatography and its post-column detection following derivatisation with ninhydrin. More recent techniques include pre-column derivatisation of the dipeptide with fluorogenic reagents such as σ-pthalaldehyde and separation by reverse phase HPLC. With the recent advances in liquid chromatography resulting in the improved resolution of amino acids, increased sensitivity, rapid analysis times, and small sample sizes, it appears likely that direct quantitation of ε(γ-glutamyl) lysine will be the preferred method for the future.
Similar content being viewed by others
References
Pisano, J. J., Finlayson, J. S. and Peyton, M. P., 1968. Science 160: 892–893.
Matacic, S. and Loewy, A. G., 1968. Biochem. Biophys. Res. Commun. 30: 356–362.
Lorand, L., Downey, J., Gotoh, T., Jacobsen, A. and Tokura, S., 1968. Biochem. Biophys. Res. Commun. 31: 222–230.
Harding, H. W. J. and Rogers, G. E., 1972. Biochim. Biophys. Acta 257: 37–39.
Rice, R. H. and Green, H., 1977. Cell 11: 417–422.
Williams-Ashman, H. G., Notides, A. C., Pabalan, S. S. and Lorand, L., 1972. Proc. Nat. Acad. Sci. 69: 2322–2325.
Chung, S. I., 1972. Ann. N.Y. Acad. Sci. 202: 240–255.
Folk, J. E. and Chung, S. I., 1973. Adv. Enzymol. 38: 109–191.
Abernethy, J. L., Hill, R. L. and Goldsmith, L. A., 1977. J. Biol. Chem. 252: 1837–1839.
Siefring, G. E. Jr., Apostol, A. B., Velasco, P. T. and Lorand, L., 1978. Biochemistry 17: 2598–2604.
Lorand, L., Hsu, L. K. H., Siefring, G. E. and Rafferty, N. S., 1981. Proc. Nat. Acad. Sci. 78: 1356–1360.
Hsu, L. K. H., Griffin, M., Wilson, J. and Lorand, L., 1981. Abstract. Fed. Proc. 40: (3).
Hirs, C. H. W., 1967. Methods in Enzymol. 11: 325–329.
Walsh, K. A. and Brown, J. R., 1962. Biochim. Biophys. Acta 58: 596–598.
Schweet, R., 1955. Fed. Proc., Fed. Am. Soc. Exp. Biol. 14: 277–278.
Schweet, R., 1956. Fed. Proc., Fed. Am. Soc. Exp. Biol. 15: 350–351.
Korngruth, M. L., Neidle, A. and Waelsch, H., 1963. Biochemistry 2: 740–745.
Pisano, J. J., Finlayson, J. S. and Peyton, M. P., 1969. Biochemistry 8: 871–876.
Asquith, R. S., Otterburn, M. S., Buchanan, J. H., Cole, M., Fletcher, J. C. and Gardner, K. L., 1970. Biochim. Biophys. Acta. 207: 342–348.
Birkbichler, P. J., Dowben, R. M., Matacic, S. and Loewy, A. G., 1973. Biochem. Biophys. Acta. 291: 149–155.
Griffin, M., Wilson, J. and Lorand, L., 1982. Anal. Biochem. 124: 406–414.
Narahashi, Y., 1970. Methods in Enzymol. 19: 651–664.
23. Markland, F. S. and Smith, E. L., 1971. In: The Enzymes (Boyer, P. D., ed.), 3rd ed., Vol. 3, pp. 561–608, New York and London: Academic Press.
Konigsberg, W., Goldstein, J. and Hill, R. J., 1963. J. Biol. Chem. 238: 2028–2033.
DeLange, R. J. and Smith, E. L., 1971. In: The Enzymes (Boyer, P. D., ed.), 3rd ed., Vol. 3, pp. 81–119, New York and London: Academic Press.
Hill, R. L. and Schmidt, W. R., 1962. J. Biol. Chem. 237: 389–396.
Matacic, S. and Loewy, A. G., 1966. Biochem. Biophys. Res. Commun. 24: 858–866.
Pisano, J. J., Finlayson, J. S., Peyton, M. P. and Nagai, Y., 1971. Proc. Nat. Acad. Sci. 68: 770–772.
Pisano, J. J., Bronzert, T. J., Peyton, M. P. and Finlayson, J. S., 1972. Ann. N.Y. Acad. Sci. 202: 98–113.
Matacic, S. S. and Loewy, A. G., 1979. Biochim. Biophys. Acta. 576: 263–268.
Sugawara, K., 1977. In: Bioch. Cutaneous Epidermal Differ. Proc. Jpn-US Semin. (Seijii, M. and Bernstein, I. A., eds.), pp. 387–396. Baltimore: Univ. PK. Press.
Birkbichler, P. J., Carter, H. A., Orr, G. R., Conway,E. and Patterson, M. K. Jr., 1978. Biochem. Biophys. Res. Commun. 84: 232–237.
Venn, R. F., Basford, J. M. and Curtis, C. G., 1978. Anal. Biochem. 87: 278–282.
Woods, K. R. and Wang, K. T., 1967. Biochim. Biophys. Acta. 133: 369–370.
Gray, W. R., 1972. Methods in Enzymol. 25: 121–138.
Blackburn, S. (ed.), 1978. Amino Acid Determination, 367 pages. New York: Marcel Dekker.
Rattenbury, J. M. (ed.), 1981. Amino Acid Analysis, 370 pages. New York: Ellis-Horwood.
Birkbichler, P. J., Orr, G. R., Carter, H. A. and Patterson, M. K. Jr., 1977. Biochem. Biophys. Res. Commun. 78: 1–7.
Harding, H. W. J. and Rogers, G. E., 1971. Biochemistry 10: 624–629.
Simpson, C. F. (ed.), 1976. Practical High Performance Liquid Chromatography. New York: Heyden Press.
Snyder, L. R. and Kirkland, J. J. (ed.), 1979. Introduction to Modern Liquid Chromatography, 2nd ed. New York: Wiley-Interscience.
Brown, P. R. and Krstulovic, A. M., 1979. Anal. Biochem. 99: 1–21.
Haag, A. and Langer, K., 1974. Chromatographia. 7: 659–662.
Annan, W. D., 1981. In: Amino Acid Analysis (Rattenburg, J. M., ed.), pp. 66–70. Chichester: Ellis-Horwood.
Engelhart, H., Asshauer, J., Neue, U. and Weigand, N., 1974. Anal. Chem. 46: 336–340.
Bayer, E., Grom, E., Kaltenegger, B. and Uhman, R., 1976. Anal. Chem. 48: 1106–1109.
Hill, D. W., Walters, F. H., Wilson, T. D. and Stuart, J. D., 1979. Anal. Chem. 51: 1338–1341.
Jones, B. N., Pääbo, S. and Stein, S., 1981. J. Liquid Chromat. 4: 565–586.
Griffin, M., Price, S. J. and Palmer, T., 1982. Clin. Chemica. Acta 125: 89–95.
Carpenter, K. J., 1960. Biochem. J. 77: 604–610.
Horwitz, W. (ed.), 1980. Official Methods of Analysis, 13th ed., pp. 776–777, No 43.224. Washington D.C.: Association of Official Analytical Chemists.
Kakade, M. L. and Leiner, I. H., 1969. Anal. Biochem. 27: 273–280.
Lorand, L., Ong, H. H., Lipinski, B., Rule, N. G., Downey, J. and Jacobsen, A., 1966. Biochem. Biophys. Res. Commun. 25: 629–637.
Pisano, J. J., Finlayson, J. S. and Peyton, M. P., 1969. Biochemistry 8: 871–876.
Weber, K. and Osborn, M., 1969. J. Biol. Chem. 244: 4406–4412.
Fairbanks, G., Steck, T. L. and Wallach, D. F. H., 1971. Biochemistry 10: 2606–2617.
Laemmli, U. K., 1970. Nature (London) 227: 680–685.
Bjerrum, O. J., Hawkins, M., Swanson, P., Griffin, M. and Lorand, L., 1981. J. Supramol. Struct. Cell. Biochem. 16: 289–301.
Soria, A., Soria, C. and Boulard, C., 1975. Experientia. 31: 1355–1357.
Cohen, I., Young-Bandala, L., Blankenberg, T. A., Siefring, G. R. and Bruner-Lorand, J., 1979. Arch. Biochem. Biophys. 192: 100–111.
Birkbichler, P. J. and Patterson, M. K., 1978. Ann. N.Y. Acad. Sci. 3: 54–65.
Mosher, D. F., 1975. J. Biol. Chem. 250: 6614–6621.
Mosher, D. F., Schad, P. E. and Kleinman, H. K., 1979. J. Clin. Invest. 64: 781–787.
Fesus, L., Falus, A., Erdei, A. and Laki, K., 1981. J. Cell Biology 89: 706–710.
Williams-Ashman, H. G., Beil, R. E., Wilson, J. H., Hawkins, M., Grayhack, J., Zunamon, A. and Weinstein, N. K., 1980. Advances in Enzyme Regulation 18: 239–258.
Lorand, L., Weissmann, L. B., Epel, D. L. and Bruner-Lorand, J., 1976. Proc. Nat. Acad. Sci. 73: 4479–4481.
Steck, T. L. and Yu, J., 1973. J. Supramol. Struct. 1: 220–232.
Lorand, L. and Ong, H. H., 1976. Biochemistry 5: 1747–1753.
Lorand, L., Chenoweth, D. and Gray, A., 1972. Ann. N.Y. Acad. Sci. 202: 155–171.
Dutton, A. and Singer, J. J., 1975. Proc. Nat. Acad. Sci. 72: 2568–2571.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Griffin, M., Wilson, J. Detection of ε(γ-glutamyl) lysine. Mol Cell Biochem 58, 37–49 (1984). https://doi.org/10.1007/BF00240603
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00240603