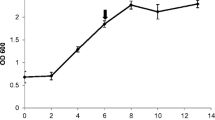
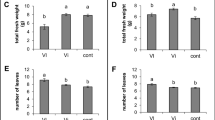
Abstract
Commercial chitosan and laminarin, as well as β-glucans, isolated from either Phytophthora megasperma f.sp. glycinea or Saccharomyces cerevisiae, were applied to decapitated tomato (Lycopersicon esculentum Mill.) plants and evaluated for their potential to induce defense mechanisms in root tissues infected by Fusarium oxysporum f.sp. radicis-lycopersici. A significant decrease in disease incidence was monitored in elicitor-treated plants as compared to water-treated plants. No difference was detected in the capacity of the elicitors under study to confer enhanced protection against pathogen attack. Ultrastructural investigations of the infected root tissues from watertreated (control) plants showed a rapid colonization of all tissues including the vascular stele. Fungal ingress was lways associated with marked host cell disorganization and cell wall alteration. In root tissues from elicitortreated plants, restriction of fungal growth to the epidermis and the outer cortex, decrease in pathogen viability, and formation of numerous wall appositions at sites of attempted penetration were the main features of the hostpathogen interaction. The wall appositions were found to vary greatly in their appearance from multi-textured to multi-layered structures, from elongated deposits to hemispherical protuberances. Application of various goldcomplexed probes to root tissue sections revealed that callose, pectin and phenolic-like compounds (likely lignin) were the main components of the newly-formed barriers. By contrast, cellulose appeared confined to outer or intermediate layers resembling the host cell wall in terms of structure and architecture. In the absence of fungal challenge, the cytologically visible consequences of elicitation were restricted to a discrete deposition of electron-opaque substances in the vacuoles of some cells, and wall appositions were not detected. The key importance of fungal challenge in the elaboration of defense mechanisms is discussed in relation to the possibility that an alarm ignal provided by the pathogen itself is required for the expression of resistance in plants previously sensitized by an exogenous elicitor.
Similar content being viewed by others
Abbreviations
- AGL:
-
Aplysia gonad lectin
- FORL:
-
Fusariumoxysporum f.sp. radicis-lycopersici
References
Albersheim, P., Mühlethaler, K., Frey-Wyssling, A. (1960) Stained pectin as seen in the electron microscope. J. Biophys. Biochem. Cytol. 8, 501–506
Benhamou, N. (1992) Ultrastructural detection of β-1,3-glucans in tobacco root tissues infected by Phytophthora parasitica var. nicotianae using a gold-complexed tobacco β-1,3-glucanase. Physiol. Mol. Plant Pathol. 41, 351–370
Benhamou, N., Theriault, G. (1992) Treatment with chitosan enhances resistance of tomato plants to the crown and root rot pathogen, Fusarium oxysporum f.sp. radicis-lycopersici. Physiol. Mol. Plant Pathol. 41, 33–52
Benhamou, N., Chamberland, H., Ouellette, G.B., Pauze, F.J. (1987) Ultrastructural localization of β-1,4-d-glucans in two pathogenic fungi and in their host tissues by means of an exoglucanase-gold complex. Can. J. Microbiol. 33, 405–417
Benhamou, N., Gilboa-Garber, N., Trudel, J., Asselin, A. (1988) Introduction of a new lectin-gold complex for the ultrastructural localization of galacturonic acids. J. Histochem. Cytochem. 36, 1403–1411
Benhamou, N., Mazau, D., Grenier, J., Esquerré-Tugayé, M.T. (1991) Time-course study of the accumulation of hydroxyprolinerich glycoproteins in root cells of susceptible and resistant tomato plants infected by Fusarium oxysporum f.sp. radicis-lycopersici. Planta 184, 196–208
Benhamou, N., Fortin, J.A., Hamel, C., St Arnaud, M., Shatilla, A. (1994) Resistance responses of mycorrhizal Ri T-DNA transformed carrot roots to infection by Fusarium oxysporum f.sp. chrysanthemi. Phytopathology 84, 958–968
Berstein, M.H. (1956) Iron as a stain for nucleic acids in electron microscopy. J. Biophys. Biochem. Cytol. 2, 633–634
Blanchette, R.A. (1991) Delignification by wood-decay fungi. Annu. Rev. Phytopathol. 29, 381–398
Bonhoff, A., Reith, B., Golecki, J., Grisebach, H. (1987) Race cultivar-specific differences in callose deposition in soybean roots following infection with Phytophthora megasperma f.sp. glycinea. Planta 172, 101–105
Brammall, R.A., Higgins, V.J. (1988) A histological comparison of fungal colonization in tomato seedlings susceptible and resistant toFusarium crown and root rot disease. Can. J. Bot. 66, 915–925
Bruce, R.J., West, C.A. (1989) Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 91, 889–897
Cabib, E., Bowers, B. (1971) Chitin and yeast budding: Localization of chitin in bud scars. J. Biol. Chem. 246, 152–159
Charest, P.M., Ouellette, G.B., Pauze, F.J. (1984) Cytological observations of early infection process of Fusarium oxysporum f.sp. radicis-lycopersici. Can. J. Bot. 62, 1232–1244
Chérif, M., Benhamou, N., Menzies, J.G., Bélanger, R.R. (1992) Silicon induced resistance in cucumber plants against Pythium ultimums. Physiol. Mol. Plant Pathol. 41, 411–425
Chet, I., Hens, Y., Mitchell, R. (1967) Chemical composition of hyphal and sclerotial walls of Sclerotium rolfsii Sacc. Can. J. Microbiol. 13, 137–141
Collmer, A., Keen, N.T. (1986) The role of pectic enzymes in plant pathogenesis. Annu. Rev. Phytopathol. 24, 383–409
Dixon, R.A., Lamb, C.J. (1990) Molecular communication in interactions between plants and microbial pathogens. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41, 339–367
Frens, G., (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold solutions. Nature Phys. Sci. 241, 20–22
Fry, S.C. (1986) Polymer-bound phenols as natural substrates of peroxidases. In: Molecular and physiological aspects of plant peroxidase, pp. 169–182, Greppin, H., Penel, C., Gaspar, Th., eds., Université de Genève, Switzerland
Geiger, J.P., Rio, B., Nandris, D., Nicole, M. (1986) Laccases of Rigidoporus lignosus and Phellinus noxius; 1. Purification and some physicochemical properties. Appl. Biochem. Biotechnol. 12, 121–133
Hahlbrock, K., Scheel, D. (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 347–364
Hahn, M.G., Bucheli, P., Cervone, F., Doares, S.H., O'Neill, R.A., Darvill, A., Albersheim, P. (1989) The roles of cell wall constituents in plant-pathogen interactions. In: Plant-microbe interactions, vol. 3, pp. 131–181, Nester, E., Kosuge, T. eds. Mc Graw-Hill, New York
Jarvis, W.R. (1988) Fusarium crown and root rot of tomatoes. Phytoprotection 69, 49–64
Kúc, J., (1987) Plant immunization and its applicability for disease control. In: Innovative approaches to plant disease control, pp. 255–274, Chet, L., ed. John Wiley & Sons, New York
Mayer, A.M. (1987) Polyphenol oxidases in plants: recent progress. Phytochemistry 26, 11–20
Minor, J.L. (1991) Location of lignin-bonded polysaccharides. J. Wood Chem. Technol. 11, 159–169
Ricci, P., Bonnet, P., Huet, J.C., Sallantin, M., Beauvais-Cante, F., Bruneteau, M., Billard, V., Michel, G., Pernollet, J.C. (1989) Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur. J. Biochem. 183, 555–563
Ride, J.P. (1983) Cell wall and other structural barriers in defense. In: Biochemical plant pathology, pp. 215–235, Callow, J.A. ed. John Wiley & Sons, New York
Ride, J.P., Barber, M.S. (1987) The effects of various treatments on induced lignification and resistance of wheat to fungi. Physiol. Mol. Plant Pathol. 31, 349–360
Robb, J., Brisson, J.D., Busch, L., Lu, B.C. (1978) Ultrastructure of wilt syndrome caused by Verticillium dahliae. Attempted localization of phenolic compounds in the vascular region. Can. J. Bot. 56, 2594–2612
Ryerson, D.E., Heath, M.C. (1992) Fungal elicitation of wall modification in leaves of Phaseolus vulgaris L. cv. Pinto. II. Effect of fungal wall components. Physiol. Mol. Plant Pathol. 40, 283–298
Sharp, J.K., Valent, B., Albersheim, P. (1984) Purification and partial characterization of a β-glucan fragment that elicits phytoalexin accumulation in soybean. J. Bio. Chem. 259, 11312–11320
Sherwood, R.T., Vance, C.P. (1976) Histochemistry of papillae formed in reed canarygrass leaves in response to noninfecting pathogenic fungi. Phytopathology 66, 503–510
Southerton, S.G., Deverall, B.J. (1990). Changes in phenolic acid level in wheat leaves expressing resistance to Puccinia recondita f.sp. tritici. Physiol.Mol.Plant Pathol. 37, 437–450
Walton, J.D. (1994) Deconstructing the cell wall. Plant Physiol. 104, 1113–1118
Author information
Authors and Affiliations
Additional information
The authors wish to thank Sylvain Noël for excellent technical assistance and Drs. J.P. Geiger and Michel Nicole (ORSTOM, Montpellier, France) for providing the purified laccase. This work was supported by a grant from the FCAR-CQVB (Fonds Québécois pour la Formation de Chercheurs et l'Aide à la Recherche and Centre Québécois de Valorisation de la Biomasse) and by a contract from the Company Tourbières Premier Ltée, Rivière-du-Loup, Québec.
Rights and permissions
About this article
Cite this article
Benhamou, N., Lafontaine, P.J. Ultrastructural and cytochemical characterization of elicitor-induced structural responses in tomato root tissues infected by Fusarium oxysporum f.sp. radicis-lycopersici . Planta 197, 89–102 (1995). https://doi.org/10.1007/BF00239944
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00239944