Summary
Stimulating electrodes were chronically implanted in the ventral hippocampal commissure and the entorhinal cortex or angular bundle of rats. Moveable metal microelectrodes which could be passed through the hippocampus were implanted. All hippocampal units were classified as complex-spike cells or theta cells on the basis of the form of their action potentials and their rates of firing in various behaviors. Field potentials and unit firing evoked from the stimulating electrodes were recorded during slow wave sleep.
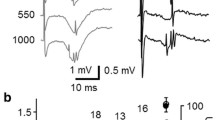
Complex-spike cells (1) could often be antidromcally activated in CA3 (it was not attempted in CA1); (2) could only be induced to fire one or two action potentials in response to a single stimulus; (3) had action potentials at the same time as the local population-spike and, in condition-test studies, were depressed when the population-spike was depressed. (The population-spike is presumably the summed synchronous action potentials of pyramidal cells.)
Theta cells: (1) were antidromically activated in only one out of 25 cases; (2) usually could fire long bursts of action potentials in response to a sufficiently intense single stimulus; (3) this firing occurred before, during, and after the local orthodromic population-spike.
Most complex-spike cells in Ammon's horn must be pyramidal cells (projection cells), and vice versa. The case for theta cells is more difficult. Some are non-pyramidal cells with locally ramifying axons, but at least some are projection cells. The data is consistent with most of them being inhibitory interneurons, but this is not established.
Similar content being viewed by others
References
Alger BE, Teyler TJ (1977) A monosynaptic fiber track studied in vitro. Evidence of a hippocampal CA1 associational system? Brain Res Bull 2: 355–365
Andersen P (1975) Organization of hippocampal neurons and their interconnections. In: Isaacson RL, Pribram KH (eds) The hippocampus, vol 1. Plenum Press, New York, pp 155–175
Andersen P, Blackstad TW, Lømo T (1966) Location and identification of excitatory synapses on hippocampal pyramidal cells. Exp Brain Res 1: 236–248
Andersen P, Bliss TVP, Skrede KK (1971) Unit analysis of hippocampal population spikes. Exp Brain Res 13: 208–221
Andersen P, Eccles JC, Loyning Y (1964a) Location of postsynaptic inhibitory synapses on hippocampal pyramids. J Neurophysiol 27: 592–607
Andersen P, Eccles JC, Loyning Y (1964b) Pathway of postsynaptic inhibition in the hippocampus. J Neurophysiol 27: 608–619
Andersen P, Gross GN, Lømo T, Sveen O (1969) Participation of inhibitory and excitatory interneurons in the control of hippocampal cortical output. In: Brazier MAB (ed) The interneuron. UCLA Forum Medical Science 11. University of California Press, Berkeley Los Angeles, pp 415–465
Bliss TVP, Gardner-Medwin AR (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the unanesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 232: 357–374
Chronister RB, De France JF (1979) Organization of projection neurons of the hippocampus. Exp Neurol 66: 509–523
Dingledine R, Langmoen IA (1980) Conductance changes and inhibitory actions of hippocampal recurrent IPSP's. Brain Res 185: 277–287
Douglas RM, Goddard GV (1975) Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res 86: 205–215
Eccles JC, Eccles RM, Lundberg A (1960) Types of neurone in and around the intermediate nucleus of the lumbosacral cord. J Physiol (Lond) 154: 89–114
Finch DM, Babb TL (1977) Response decrement in a hippocampal basket cell. Brain Res 130: 354–359
Fox SE (1978) Hippocampal electrophysiology in freely-moving rats. Doctoral thesis, University of Michigan, Ann Arbor
Fox SE, Ranck JB, Jr (1975) Localization and anatomical identification of theta and complex spike cells in dorsal hippocampal formation of rats. Exp Neurol 49: 299–313
Fox SE, Ranck JB, Jr (1977) Hippocampal complex-spike and theta cell activity evoked by stimulation of limbic structures in unrestrained rats. Neurosci Abstr 3: 198
Fox SE, Ranck JB, Jr (1979) Hippocampal field potentials evoked by stimulation of multiple limbic structures in freely moving rats. Neuroscience 4: 1467–1478
Fuller JH, Schlag JD (1976) Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res 112: 283–298
Fugita Y (1979) Evidence for the existence of inhibitory postsynaptic potentials in dendrites and their functional significance in hippocampal pyramidal cells of adult rabbits. Brain Res 175: 59–69
Lømo T (1971) Potentiation of monosynaptic EPSPs in the perforant path-dentate granule cell synapse. Exp Brain Res 12: 46–63
Lorente de Nó R. (1934) Studies on the structure of the cerebral cortex. II. Continuation of the study of the Ammonic system. J Psychol Neurol (Leipz) 46: 113–177
Purpura DP, Prelevic S, Santini M (1968) Postsynaptic potentials and spike variations in the feline hippocampus during postnatal ontogenesis. Exp Neurol 22: 408–422
Ramón y Cajal S (1968) The structure of ammon's horn. Thomas, Springfield
Ranck JB, Jr (1973a) A moveable microelectrode for recording from single neurons in unrestrained rats. In: Phillips MI (ed) Brain unit activity during behavior. Thomas, Springfield, pp 76–79
Ranck JB, Jr (1973b) Studies on single neurons in dorsal hippocampal formation and septum of unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol 41: 461–555
Schwartzkroin PA, Mathers LH (1978) Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res 157: 1–10
Spencer WA, Kandel ER (1961) Hippocampal neuron responses to selective activation of recurrent collaterals of hippocampofugal axons. Exp Neurol 4: 149–161
Steward O, White WF, Cotman CW, Lynch G (1976) Potentiation of excitatory synaptic transmission in the normal and in the reinnervated dentate gyrus of the rat. Exp Brain Res 26: 423–441
Winson J, Abzug C (1978) Neuronal transmission through hip-pocampal pathways dependent on behavior. J Neurophysiol 41: 716–732
Author information
Authors and Affiliations
Additional information
This work was supported in part by Grants NS 12664 and NS 14497 from the National Institutes of Health and BNS 77-09375 from the National Science Foundation to J.B. Ranck, Jr. and N.I.H. Grant NS 10987 to VE. Amassian. The results have been presented in preliminary form elsewhere (Fox and Ranck 1977; Fox 1978)
Rights and permissions
About this article
Cite this article
Fox, S.E., Ranck, J.B. Electrophysiological characteristics of hippocampal complex-spike cells and theta cells. Exp Brain Res 41, 399–410 (1981). https://doi.org/10.1007/BF00238898
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00238898