Summary
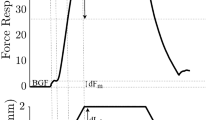
Flexor (tibialis anterior, TA, and extensor digitorum longus, EDL) and extensor (soleus, SOL) muscles in the decerebrate cat were subjected to length changes and the force responses were measured. Resultant muscular stiffness, which arises from the mechanical reaction of muscle fibers contracting prior to the length change and from a change in force due to reflex action, was calculated by dividing the changes in force by the corresponding length changes. As shown previously in the premammillary preparation, resultant stiffness was usually higher in SOL than in TA or EDL. Following an intercollicular transection in some preparations, resultant stiffness increased markedly for TA but not substantially for SOL. During continuous electrical stimulation in the magnocellular red nucleus in premammillary preparations, resultant stiffness of SOL decreased for a wide range of forces while EDL responses were unaffected. These results show that reflex gain is not determined solely by the level of motoneuronal excitability but also by a descending control from the brainstem, and that the lower resultant stiffness in flexors compared to extensors in the decerebrate cat is set by this control system and not by inherent differences in the strength of autogenetic reflex pathways for the two muscles.
Similar content being viewed by others
References
Akazawa K, Aldridge JW, Steeves JD, Stein RB (1982) Modulation of stretch reflexes during locomotion in the mesencephalic cat. J Physiol (Lond) 329: 553–567
Anden N-B, Jukes MGM, Lundberg A, Vyklicky L (1966) The effect of DOPA on the spinal cord. I. Influence on transmission from primary afferents. Acta Physiol Scand 67: 373–386
Appelberg B, Jeneskog T, Johansson H (1975) Rubrospinal control of static and dynamic fusimotor neurones. Acta Physiol Scand 95: 431–440
Appelberg B, Molander C (1967) A rabro-olivary pathway. I.Identification of a decending system for control of the dynamic sensitivity of muscle spindles. Exp Brain Res 3: 373–381
Baldissera F, Lundberg A, Udo M (1972) Stimulation of pre- and postsynaptic elements in the red nucleus. Exp Brain Res 15: 151–167
Baldissera F, Hultborn H, Illert M (1981) Integration in spinal neuronal systems. In: Brooks VB (ed) The nervous system, Vol II, Motor control, Part 1. American Physiological Society, Bethesda (Handbook of physiology, Sect 1, Vol II, pp 509–596)
Engberg I, Lundberg A, Ryall RW (1968) Reticulospinal inhibition of transmission in reflex pathways. J Physiol 194: 201–223
Feldman AG (1966) Functional tuning of the nervous system during control of movement or maintenance of a steady posture. III. Mechanographic analysis of the execution by man of the simplest motor tasks. Biophysics 11: 766–775
Feldman AG (1980) Superposition of motor programs. I. Rhythmic forearm movements in man. Neuroscience 5: 81–90
Feldman AG, Orlovsky GN (1972) The influence of different descending systems on the tonic stretch reflex in the cat. Exp Neurol 37: 481–494
Feldman AG (1982) Voluntary control of muscle length and tension, independently controlled variables, and invariant length-tension curves. Behav Brain Sci 5: 545–546
Forssberg H (1979) Stumbling corrective reaction: a phasedependent compensatory reaction during locomotion. J Neurophysiol 42: 936–953
Forssberg H, Grillner S, Rossignol S (1977) Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Res 132: 121–139
Goslow GE Jr, Reinking RM, Stuart DG (1973) The cat step cycle: hindlimb joint angles and muscle lengths during unrestrained locomotion. J Morphol 141: 1–42
Henneman E, Clamann HP, Gillies JD, Skinner RD (1974) Rank order of motoneurons within a pool: law of combination. J Neurophysiol 37: 1338–1349
Hoffer JA, Andreassen S (1981) Regulation of soleus muscle stiffness in premammillary cats: mechanical and reflex components. J Neurophysiol 45: 267–285
Hoffer JA, Leonard TR, Spence NL, Cleland CL (1984) Reflex gain, muscle stiffness and viscosity in normal cats. Soc Neurosci Abstr 10: 330
Hongo T, Jankowska E, Lundberg A (1969a) The rubrospinal tract. I. Effects on alpha-motoneurones innervating hindlimb muscles in the cat. Exp Brain Res 7: 344–364
Hongo T, Jankowska E, Lundberg A (1969b) The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res 7: 365–391
Houk JC (1972) The phylogeny of muscular control configurations. In: Drischel H, Dettmar A (eds) Biocybernetics, Vol 4. Fischer, Jena, pp 125–144
Houk JC, Singer JJ, Goldman MR (1970) An evaluation of length and force feedback to soleus muscles of decerebrate cats. J Neurophysiol 33: 784–811
Houk JC, Rymer WZ (1981) Neural control of muscle length and tension. In: Brooks VB (ed) The nervous system, Vol II, Motor control, Part 1. American Physiological Society, Bethesda (Handbook of physiology, Sect 1, Vol II, pp 509–596)
Hulliger M, Matthews PBC, Noth J (1977) Static and dynamic fusimotor action on the response of Ia fibres to low frequency sinusoidal stretching of widely ranging amplitude. J Physiol (Lond) 267: 811–838
Jeneskog T (1974) Parallel activation of dynamic fusimotor neurones and a climbing fibre system from the cat brain stem. I. Effects from the rubral region. Acta Physiol Scand 91: 223–242
Jeneskog T, Johansson H (1977) The rubro-bulbospinal path. A descending system known to influence dynamic fusimotor neurones and its interaction with distal cutaneous afferents in the control of flexor reflex afferent pathways. Exp Brain Res 27: 161–179
Liddell EGT, Sherrington CS (1924) Reflexes in response to stretch (myotatic reflexes). Proc R Soc Lond B 96: 212–242
Matthews PBC (1959) A study of certain factors influencing the stretch reflex of the decerebrate cat. J Physiol 147: 547–564
Merrill EG, Ainsworth A (1972) Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng 10: 662–672
Nichols TR (1973) Reflex and non-reflex stiffness of the soleus muscle in the cat. In: Stein RB, Pearson KG, Smith RS, Redford JB (eds) Control of posture and locomotion. Plenum, New York, pp 407–410
Nichols TR (1974) Soleus muscle stiffness and its reflex control. PhD Thesis, Cambridge, Harvard University
Nichols TR (1981) Evidence for authentic changes in the gain of an autogenetic reflex in the soleus muscle in the decerebrate cat. Soc Neurosci Abstr 7: 688
Nichols TR (1983) Reflex action in an agonist-antagonist muscle system in the decerebrate cat. Soc Neurosci Abstr 9: 527
Nichols TR, Houk JC (1973) Reflex compensation for variations in the mechanical properties of a muscle. Science 181: 182–184
Nichols TR, Houk JC (1976) Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol 39: 119–142
Nichols TR (1985a) Autogenetic reflex action in tibialis anterior compared with that in soleus muscle in the decerebrate cat. Exp Brain Res 59: 232–241
Nichols TR (1985b) Mechanical analysis of “antagonogenic” reflex action in decerebrate cats. Soc Neurosci Abstr 11: 213
Pollock LJ, Davis L (1930) The reflex activities of a decerebrate animal. J Comp Neurol 50: 377–411
Rack PMH, Westbury DR (1969) The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol 204: 443–460
Schwindt PC (1981) Control of motoneuron output by pathways descending from the brain stem. In: Towe AL, Luschei ES (eds) Motor coordination. Plenum, New York London (Handbook of behavioral neurobiology, Vol 5, pp 139–230)
Shefchyk SJ, Stein RB, Jordan LM (1984) Synaptic transmission from muscle afferents during fictive locomotion in the mesencephalic cat. J Neurophysiol 51: 986–997
Wolpaw JR, O'Keefe JA, Dowman R (1984) Adaptive plasticity in primate spinal stretch reflex (SSR): a two-phase process. Soc Neurosci Abstr 10: 129
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nichols, T.R., Steeves, J.D. Resetting of resultant stiffness in ankle flexor and extensor muscles in the decerebrate cat. Exp Brain Res 62, 401–410 (1986). https://doi.org/10.1007/BF00238859
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00238859