Summary
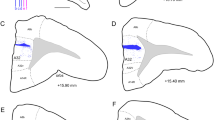
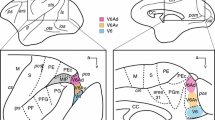
Efferent cortical connections of the cingulate gyrus are investigated in rhesus monkey using autoradiographic technique. The results indicate that the rostralmost part of the cingulate gyrus (area 32) sends projections to the lateral prefrontal and midorbitofrontal cortex and to the rostral portion of the superior temporal gyrus. In contrast, the other two major subdivisions of the cingulate gyrus, areas 24 and 23, have widespread connections within the cortex. Area 24, for example, projects to the pre-motor region (areas 6 and 8), the fronto-orbital cortex (area 12), the rostral part of the inferior parietal lobule, the anterior insular cortex, the perirhinal area and the laterobasal nucleus of amygdala. Area 23, likewise, sends its connections to the dorsal prefrontal cortex (areas 9 and 10), the rostral orbital cortex (area 11), the parieto-temporal cortex (posterior part of the inferior parietal lobule and the superior temporal sulcus), the parahippocampal gyrus (areas TH and TF), the retrosplenial region and the presubiculum. It seems that the connections of the rostralmost part of the cingulate gyrus resemble the efferent cortical connectional patterns described for lateral prefrontal and orbito-frontal cortex, whereas the projections of areas 24 and 23 are directed to the neocortical, the paralimbic and the limbic areas.
Similar content being viewed by others
References
Adey WR, Meyer M (1952) An experimental study of hippocampal afferent pathways from prefrontal and cingulate areas in the monkey. J Anat 86: 58–74
Baleydier C, Mauguiere F (1980) The duality of the cingulate gyrus in monkey; neuroanatomical study and functional hypothesis. Brain 103: 525–559
Bignall KE, Imbert M (1969) Polysensory and cortico-cortical projections to frontal lobe of squirrel and rhesus monkeys. Electroencephalogr Clin Neurophysiol 26: 206–215
Bonin G von, Bailey P (1947) The neocortex of macaca mulatta. University of Illinois Press, Urbana
Brodmann K (1909) Vergleichende Lokalisationslehre der Großhirnrinde. Barth, Leipzig
Chavis DA, Pandya DN (1976) Further observation on corticofrontal connections in rhesus monkey. Brain Res 117: 369–386
Cowan WM, Gottlieb DI, Hendrickson AE, Price JL, Woolsey TA (1972) The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res 37: 21–51
Critchley M (1949) The phenomenon of tactile inattention with special reference to parietal lesions. Brain 72: 538–561
Denny-Brown D, Chambers RA (1958) The parietal lobe and behavior. Res Publ Assoc Res Nerv Ment Dis 36: 35–117
Denny-Brown D, Meyer JS, Horenstein S (1953) The significance of perceptual rivalry resulting from parietal lesions. Brain 75: 433–471
Desimone R, Gross CG (1979) Visual areas in the temporal cortex of the macaque. Brain Res 178: 363–380
Desiraju T (1976) Electrophysiology of frontal granular cortex. III. The cingulate-prefrontal relation in primate. Brain Res 109: 473–485
Domesick VB (1969) Projections from the cingulate cortex in the rat. Brain Res 12: 296–320
Domesick VB (1972) Thalamic relationships of the medial cortex in the rat. Brain Behav Evol 6: 457–483
Economo CV, Koskinas GM (1925) Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. Springer, Berlin
Eidelberg E, Schwartz AJ (1971) Experimental analysis of extinction phenomenon in monkey. Brain 94: 91–188
Fallaice LA, Allen RP, McQueen JD, Northrup B (1971) Cognitive deficits from bilateral cingulotomy for intractable pain in man. Dis Nerv Syst 32: 171–175
Fedio P, Ommaya AK (1970) Bilateral cingulum lesions and stimulation in man with lateralized impairment in short-term memory. Exp Neurol 29: 84–91
Heilman KM, Pandya DN, Geschwind N (1970) Trimodal inattention following parietal lobe ablations. Trans Am Neurol Assoc 95: 259–261
Jacobson S, Trojanowski JQ (1977) Prefrontal granular cortex of the rhesus monkey. I. Intrahemispheric cortical afferents. Brain Res 132: 209–233
Jones EG, Powell TPS (1970) An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain 93: 793–820
Kaada BR, Pribram JA, Epstein J (1949) Respiratory and vascular responses in monkeys from temporal pole, insular, orbital surfaces and cingulate gyrus. J Neurophysiol 12: 347–356
Kali KY (1973) Connections of the cingulate cortex in the cat. Doctoral Dissertation
Kemper TL, Wright SJ, Locke S (1972) Relationship between the septum and cingulate gyrus. J Comp Neurol 146: 465–478
Krettek JE, Price JL (1977) Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol 172: 687–722
Krieg WJS (1963) Connection of the cerebral cortex. Brain Books, Evanston, pp 5–374
Larsons SJ (1962) The efferent connections of the cingulate gyrus in the magaque. Anat Rec 142: 251
Locke S, Angevine JB, Yakovlev PI (1964) Thalamocortical projection of the lateral dorsal nucleus in cat and monkey. Arch Neurol 11: 1–12
MacLean PD (1949) Psychosomatic disease and the ‘visceral brain”: recent developments bearing on the Papez theory of emotion. Psychosom Med 11: 338–353
McLardy T (1971) Anticipatory recall deficit after cingulotomy in rats. Exp Neurol 32: 141–151
Meibach RC, Siegel A. (1977) Subicular projections to the posterior cingulate cortex in rats. Exp Neurol 57: 264–270
Mesulam MM, Geschwind N (1978) On the possible role of the neocortex and its connections in the process of attention and schizophrenia: clinical cases of inattention in man and experimental anatomy in monkey. J Psychiat Res 14: 249–259
Mesulam MM, Van Hoesen GW, Pandya DN, Geschwind N (1977) Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: A study with a new method for horseradish peroxidase histochemistry. Brain Res 136: 393–414
Mufson EJ, Mesulam MM, Pandya DN (1979) Insular cortex and amygdala have reciprocal connections in the rhesus monkey. Soc Neurosci 5: 280
Nauta WJH (1961) Fibre degeneration following lesions of the amygdaloid complex in the monkey. J Anat 95: 515–531
Nauta WJH (1964) Some efferent connections of the prefrontal cortex in the monkey. In: Warren JM, Akert K (eds) The frontal granular cortex and behavior. McGraw-Hill, New York, pp 397–409
Nelson CN, Bignall KE (1973) Interactions of sensory and nonspecific thalamic inputs to cortical poly-sensory units in squirrel monkey. Exp Neurol 40: 189–206
Niimi K, Niimi M, Okada Y (1978) Thalamic afferents to limbic cortex in the cat studied with the method of retrograde axonal transport of horseradish peroxidase. Brain Res 154: 225–238
Niki H, Watanabe M (1976) Cingulate unit activity and delayed response. Brain Res 110: 381–386
Pandya DN, Domesick VB, Van Hoesen GW, Mesulam MM (1972) Projections of the cingulate gyms and cingulum in the rhesus monkey. Anat Rec 172: 379
Pandya DN, Dye P, Butters N (1971) Efferent cortico-cortical projections of the prefrontal cortex in the rhesus monkey. Brain Res 31: 35–46
Pandya DN, Kuypers HGJM (1969) Cortico-cortical connections in the rhesus monkey. Brain Res 13: 13–36
Pandya DN, Van Hoesen GW, Mesulam MM (1979) The cortical projections of the cingulate gyrus in the rhesus monkey. Anat Rec 193: 643–644
Papez JW (1937) A proposed mechanism of emotion. Arch Neurol Psychiatr 38: 725–733
Petras JM (1971) Connections of the parietal lobe. J Psychiatr Res 8: 189–201
Petrides M, Iversen SD (1978) The effect of selective anterior and posterior association cortex lesions in the monkey performance of a visual-auditory compound discrimination test. Neuropsychologia 16: 527–537
Rosene DL, Van Hoesen GW (1977) Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science 198: 315–317
Sanides F (1972) Representation in the cerebral cortex and its areal lamination pattern. In: Bourne GH (ed) The structure and function of nervous tissue. Academic Press, New York, vol 5, pp 329–453
Seltzer B, Pandya DN (1976) Some cortical projections to the parahippocampal area in the rhesus monkey. Exp Neurol 50: 146–160
Seltzer B, Pandya DN (1978) Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res 149: 1–24
Seltzer B, Van Hoesen GW (1979) A direct inferior parietal lobule projection to the presubiculum in the rhesus monkey. Brain Res 179: 157–161
Shipley MT (1975) The topographic and laminar organization of the presubiculum's projection to the ipsi- and contralateral entorhinal cortex in the guinea pig. J Comp Neurol 160: 127–146
Showers MJC (1959) The cingular gyrus: Additional motor area and cortical autonomie regulator. J Comp Neurol 112: 231–301
Siegel A, Chabora J (1971) Effects of electrical stimulation of the cingulate gyrus upon attack behavior elicited from the hypothalamus in the cat. Brain Res 32: 169–177
Simon EJ, Hiller JM (1978) In vitro studies on opiate receptors and their ligands. Fed Proc Am Soc Exp Biol 37: 141–146
Talairach J, Bancaud F, Geir S, Borda-Ferrer M, Bonis A, Szikla G, Rush M (1973) The cingulate gyrus and human behavior. Electroencephalogr Clin Neurophysiol 34: 45–52
Thomas GJ, Hostetter G, Baker DJ (1968) Behavioral function of the limbic system. Prog Physiol Psychol 2: 230–311
Van Hoesen GW, Pandya DN (1975) Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res 95: 39–59
Van Hoesen GW, Pandya DN, Butters N (1972) Cortical afferents to the entorhinal cortex of the rhesus monkey. Science 175: 1471–1473
Van Hoesen GW, Vogt BA, Pandya DN, McKenna T (1980) Compound stimulus differentiation following periacurate ablations in the rhesus monkey. Brain Res 168: 365–378
Vogt BA (1976) Retrosplenial cortex in the rhesus monkey: a cytoarchitectonic and Golgi study. J Comp Neurol 1969: 63–98
Vogt BA, Rosene DL, Pandya DN (1979) Thalamic and cortical afferents differentiate anterior and posterior cingulate cortex in the monkey. Science 204: 205–207
Walker AE (1940) A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol 73: 59–86
Ward AA (1948) The angular gyrus; Area 24. J Neurophysiol 11: 13–23
Watson RT, Heilman KM, Cauthen JC, King FA (1973) Neglect after cingulectomy. Neurology (Minneap) 23: 1003–1007
Welch K, Stuteville P (1958) Experimental production of unilateral neglect in monkeys. Brain 8: 342–347
Whitty CWM (1966) Some early and transient changes in psychological function after anterior cingulatomy in man. Int J Neurol 3: 413–490
Yakovlev PI, Locke S (1961) Limbic nuclei of the thalamus and connections of limic cortex. III. Corticocortical connections of the anterior cingulate gyrus, the cingulum, and the subcallosal bundle in monkey. Arch Neurol 5: 364–400
Yakovlev PI, Locke S, Koskoff DY, Patton RA (1960) Limbic nuclei of the thalamus and connections of limbic cortex. I. Organization of the projections of the anterior group of nuclei and of the midline nuclei of the thalamus to the anterior cingulate gyrus and hippocampal rudiment in the monkey. Arch Neurol 3: 620–641
Author information
Authors and Affiliations
Additional information
This study was in part supported by NIH Grant NS09211 and V.A. Research Project No. 6901
Preliminary results of this investigation were presented in abstract form (Pandya et al. 1979)
Rights and permissions
About this article
Cite this article
Pandya, D.N., Van Hoesen, G.W. & Mesulam, M.M. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp Brain Res 42, 319–330 (1981). https://doi.org/10.1007/BF00237497
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00237497